Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons
- PMID: 10921879
- PMCID: PMC306606
- DOI: 10.1093/emboj/19.15.3978
Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons
Abstract
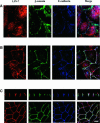
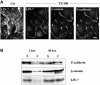
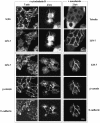
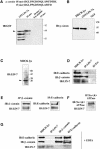
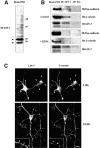
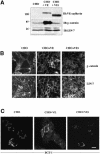
The heterotrimeric PDZ complex containing LIN-2, LIN-7 and LIN-10 is known to be involved in the organization of epithelial and neuronal junctions in Caenorhabditis elegans and mammals. We report here that mammalian LIN-7 PDZ proteins form a complex with cadherin and beta-catenin in epithelia and neurons. The association of LIN-7 with cadherin and beta-catenin is Ca(2+) dependent and is mediated by the direct binding of LIN-7 to the C-terminal PDZ target sequence of beta-catenin, as demonstrated by means of co-immunoprecipitation experiments and in vitro binding assays with the recombinant glutathione S-transferase:LIN-7A. The presence of beta-catenin at the junction is required in order to relocate LIN-7 from the cytosol to cadherin-mediated adhesions, thus indicating that LIN-7 junctional recruitment is beta-catenin dependent and that one functional role of the binding is to localize LIN-7. Moreover, when LIN-7 is present at the beta-catenin-containing junctions, it determines the accumulation of binding partners, thus suggesting the mechanism by which beta-catenin mediates the organization of the junctional domain.
Figures
References
-
- Borg J.P., Straight,S.W., Kaech,S.M., de Taddeo-Borg,M., Kroon,D.E., Karnak,D., Turner,R.S., Kim,S.K. and Margolis,B. (1998) Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem., 273, 31633–31636. - PubMed
-
- Butz S., Okamoto,M. and Sudhof,T.C. (1998) A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell, 94, 773–782. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

