Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes
- PMID: 10921880
- PMCID: PMC306586
- DOI: 10.1093/emboj/19.15.3990
Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes
Abstract
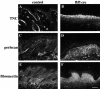
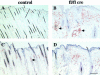
beta 1 integrins are ubiquitously expressed receptors that mediate cell-cell and cell-extracellular matrix interactions. To analyze the function of beta1 integrin in skin we generated mice with a keratinocyte-restricted deletion of the beta 1 integrin gene using the cre-loxP system. Mutant mice developed severe hair loss due to a reduced proliferation of hair matrix cells and severe hair follicle abnormalities. Eventually, the malformed hair follicles were removed by infiltrating macrophages. The epidermis of the back skin became hyperthickened, the basal keratinocytes showed reduced expression of alpha 6 beta 4 integrin, and the number of hemidesmosomes decreased. Basement membrane components were atypically deposited and, at least in the case of laminin-5, improperly processed, leading to disruption of the basement membrane and blister formation at the dermal-epidermal junction. In contrast, the integrity of the basement membrane surrounding the beta 1-deficient hair follicle was not affected. Finally, the dermis became fibrotic. These results demonstrate an important role of beta 1 integrins in hair follicle morphogenesis, in the processing of basement membrane components, in the maintenance of some, but not all basement membranes, in keratinocyte differentiation and proliferation, and in the formation and/or maintenance of hemidesmosomes.
Figures












References
-
- Aumailley M. and Rousselle,P. (1999) Laminins of the dermo-epidermal junction. Matrix Biol., 18, 19–28. - PubMed
-
- Bagutti C., Wobus,A.M., Fässler,R. and Watt,F.M. (1996) Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev. Biol., 179, 184–196. - PubMed
-
- Bottazzi M.E. and Assoian,R.K. (1997) The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol., 7, 348–352. - PubMed
-
- Brakebusch C., Hirsch,E., Potocnik,A. and Fässler,R. (1997) Genetic analysis of β1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J. Cell Sci., 110, 2895–2904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

