TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha
- PMID: 10921895
- PMCID: PMC306595
- DOI: 10.1093/emboj/19.15.4154
TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha
Abstract
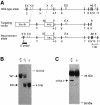
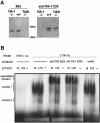
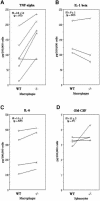
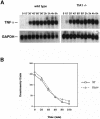
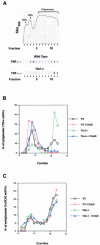
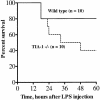
TIA-1 and TIAR are related proteins that bind to an AU-rich element (ARE) in the 3' untranslated region of tumor necrosis factor alpha (TNF-alpha) transcripts. To determine the functional significance of this interaction, we used homologous recombination to produce mutant mice lacking TIA-1. Although lipopolysaccharide (LPS)-stimulated macrophages derived from wild-type and TIA-1(-/-) mice express similar amounts of TNF-alpha transcripts, macrophages lacking TIA-1 produce significantly more TNF-alpha protein than wild-type controls. The half-life of TNF-alpha transcripts is similar in wild-type and TIA-1(-/-) macrophages, indicating that TIA-1 does not regulate transcript stability. Rather, the absence of TIA-1 significantly increases the proportion of TNF-alpha transcripts that associate with polysomes, suggesting that TIA-1 normally functions as a translational silencer. TIA-1 does not appear to regulate the production of interleukin 1 beta, granulocyte-macrophage colony-stimulating factor or interferon gamma, indicating that its effects are, at least partially, transcript specific. Mice lacking TIA-1 are hypersensitive to the toxic effects of LPS, indicating that this translational control pathway may regulate the organismal response to microbial stress.
Figures
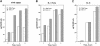
References
-
- Berlanga J., Herrero,S. and DeHaro,C. (1998) Characterization of the hemin-sensitive eukaryotic initiation factor 2α kinase from mouse nonerythroid cells. J. Cell Biol., 273, 32340–32346. - PubMed
-
- Beutler B. (1999) The role of tumor necrosis factor in health and disease. J. Rheum., 26, 16–21. - PubMed
-
- Biragyn A. and Nedospasov,S.A. (1995) Lipopolysaccharide-induced expression of TNF-α gene in the macrophage cell line ANA-1 is regulated at the level of transcription processivity. J. Immunol., 155, 674–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

