Elongation arrest is a physiologically important function of signal recognition particle
- PMID: 10921896
- PMCID: PMC306590
- DOI: 10.1093/emboj/19.15.4164
Elongation arrest is a physiologically important function of signal recognition particle
Abstract
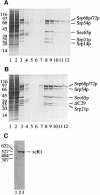
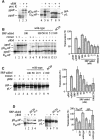
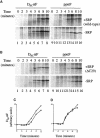
Signal recognition particle (SRP) targets proteins for co-translational insertion through or into the endoplasmic reticulum membrane. Mammalian SRP slows nascent chain elongation by the ribosome during targeting in vitro. This 'elongation arrest' activity requires the SRP9/14 subunit of the particle and interactions of the C-terminus of SRP14. We have purified SRP from Saccharomyces cerevisiae and demonstrated that it too has elongation arrest activity. A yeast SRP containing Srp14p truncated at its C-terminus (delta C29) did not maintain elongation arrest, was substantially deficient in promoting translocation and interfered with targeting by wild-type SRP. In vivo, this mutation conferred a constitutive defect in the coupling of protein translation and translocation and temperature-sensitive growth, but only a slight defect in protein translocation. In combination, these data indicate that the primary defect in SRP delta C29 is in elongation arrest, and that this is a physiologically important and conserved function of eukaryotic SRP.
Figures

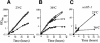

Similar articles
-
Structure of the signal recognition particle interacting with the elongation-arrested ribosome.Nature. 2004 Feb 26;427(6977):808-14. doi: 10.1038/nature02342. Nature. 2004. PMID: 14985753
-
The Alu domain homolog of the yeast signal recognition particle consists of an Srp14p homodimer and a yeast-specific RNA structure.RNA. 1999 Oct;5(10):1333-47. doi: 10.1017/s1355838299991045. RNA. 1999. PMID: 10573124 Free PMC article.
-
New insights into signal recognition and elongation arrest activities of the signal recognition particle.Biol Chem. 1999 Feb;380(2):135-45. doi: 10.1515/BC.1999.021. Biol Chem. 1999. PMID: 10195420 Review.
-
Advances in the structure and functions of signal recognition particle in protein targeting.J Biol Regul Homeost Agents. 2003 Oct-Dec;17(4):303-7. J Biol Regul Homeost Agents. 2003. PMID: 15065758 Review.
-
Signal recognition particle-dependent protein targeting, universal to all kingdoms of life.Rev Physiol Biochem Pharmacol. 2003;146:55-94. doi: 10.1007/s10254-002-0002-9. Epub 2002 Dec 17. Rev Physiol Biochem Pharmacol. 2003. PMID: 12605305 Review.
Cited by
-
Recognition of a subset of signal sequences by Ssh1p, a Sec61p-related protein in the membrane of endoplasmic reticulum of yeast Saccharomyces cerevisiae.Mol Biol Cell. 2002 Jul;13(7):2223-32. doi: 10.1091/mbc.01-10-0518. Mol Biol Cell. 2002. PMID: 12134063 Free PMC article.
-
Targeting of Proteins for Translocation at the Endoplasmic Reticulum.Int J Mol Sci. 2022 Mar 29;23(7):3773. doi: 10.3390/ijms23073773. Int J Mol Sci. 2022. PMID: 35409131 Free PMC article. Review.
-
Access to ribosomal protein Rpl25p by the signal recognition particle is required for efficient cotranslational translocation.Mol Biol Cell. 2008 Jul;19(7):2876-84. doi: 10.1091/mbc.e07-10-1074. Epub 2008 Apr 30. Mol Biol Cell. 2008. PMID: 18448667 Free PMC article.
-
Ending a bad start: Triggers and mechanisms of co-translational protein degradation.Front Mol Biosci. 2023 Jan 4;9:1089825. doi: 10.3389/fmolb.2022.1089825. eCollection 2022. Front Mol Biosci. 2023. PMID: 36660423 Free PMC article. Review.
-
Identification and comparative analysis of components from the signal recognition particle in protozoa and fungi.BMC Genomics. 2004 Jan 13;5(1):5. doi: 10.1186/1471-2164-5-5. BMC Genomics. 2004. PMID: 14720308 Free PMC article.
References
-
- Bui N. and Strüb,K. (1999) New insights into signal recognition and elongation arrest activities of the signal recognition particle. Biol. Chem., 380, 135–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

