Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy
- PMID: 10921956
- PMCID: PMC87154
- DOI: 10.1128/JCM.38.8.2943-2948.2000
Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy
Abstract
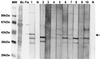
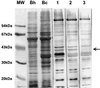
Cat scratch disease (CSD) is a frequent clinical outcome of Bartonella henselae infection in humans. Recently, two case reports indicated Bartonella clarridgeiae as an additional causative agent of CSD. Both pathogens have been isolated from domestic cats, which are considered to be their natural reservoir. B. clarridgeiae and B. henselae can be distinguished phenotypically by the presence or absence of flagella, respectively. Separation of the protein content of purified flagella of B. clarridgeiae by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis indicated that the flagellar filament is mainly composed of a polypeptide with a mass of 41 kDa. N-terminal sequencing of 20 amino acids of this protein revealed a perfect match to the N-terminal sequence of flagellin (FlaA) as deduced from the sequence of the flaA gene cloned from B. clarridgeiae. The flagellin of B. clarridgeiae is closely related to flagellins of Bartonella bacilliformis and several Bartonella-related bacteria. Since flagellar proteins are often immunodominant antigens, we investigated whether antibodies specific for the FlaA protein of B. clarridgeiae are found in patients with CSD or lymphadenopathy. Immunoblotting with 724 sera of patients suffering from lymphadenopathy and 100 healthy controls indicated specific FlaA antibodies in 3.9% of the patients' sera but in none of the controls. B. clarridgeiae FlaA is thus antigenic and expressed in vivo, providing a valuable tool for serological testing. Our results further indicate that B. clarridgeiae might be a possible etiologic agent of CSD or lymphadenopathy. However, it remains to be clarified whether antibodies to the FlaA protein of B. clarridgeiae are a useful indicator of acute infection.
Figures



Similar articles
-
Evaluation of indirect fluorescence antibody assay for detection of Bartonella clarridgeiae and Seroprevalence of B. clarridgeiae among patients with suspected cat scratch disease.J Clin Microbiol. 2004 Jul;42(7):3346-9. doi: 10.1128/JCM.42.7.3346-3349.2004. J Clin Microbiol. 2004. PMID: 15243113 Free PMC article.
-
Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes.Infect Immun. 1993 Dec;61(12):4962-71. doi: 10.1128/iai.61.12.4962-4971.1993. Infect Immun. 1993. PMID: 8225570 Free PMC article.
-
Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines.Am J Trop Med Hyg. 1999 Apr;60(4):593-7. doi: 10.4269/ajtmh.1999.60.593. Am J Trop Med Hyg. 1999. PMID: 10348234
-
[Prevalence of Bartonella henselae in stray and domestic cats in different Italian areas: evaluation of the potential risk of transmission of Bartonella to humans].Parassitologia. 2004 Jun;46(1-2):127-9. Parassitologia. 2004. PMID: 15305701 Review. Italian.
-
[Bartonella infection in humans].Presse Med. 1999 Feb 27;28(8):429-34, 438. Presse Med. 1999. PMID: 10093604 Review. French.
Cited by
-
Mixed infections, cryptic diversity, and vector-borne pathogens: evidence from Polygenis fleas and Bartonella species.Appl Environ Microbiol. 2007 Oct;73(19):6045-52. doi: 10.1128/AEM.00228-07. Epub 2007 Aug 10. Appl Environ Microbiol. 2007. PMID: 17693558 Free PMC article.
-
Experimental infection of domestic cats with Bartonella koehlerae and comparison of protein and DNA profiles with those of other Bartonella species infecting felines.J Clin Microbiol. 2002 Feb;40(2):466-74. doi: 10.1128/JCM.40.2.466-474.2002. J Clin Microbiol. 2002. PMID: 11825958 Free PMC article.
-
Phylogeny and putative virulence gene analysis of Bartonella bovis.J Vet Med Sci. 2018 Apr 18;80(4):653-661. doi: 10.1292/jvms.17-0448. Epub 2017 Dec 29. J Vet Med Sci. 2018. PMID: 29311425 Free PMC article.
-
Evaluation of indirect fluorescence antibody assay for detection of Bartonella clarridgeiae and Seroprevalence of B. clarridgeiae among patients with suspected cat scratch disease.J Clin Microbiol. 2004 Jul;42(7):3346-9. doi: 10.1128/JCM.42.7.3346-3349.2004. J Clin Microbiol. 2004. PMID: 15243113 Free PMC article.
-
Intruders below the radar: molecular pathogenesis of Bartonella spp.Clin Microbiol Rev. 2012 Jan;25(1):42-78. doi: 10.1128/CMR.05009-11. Clin Microbiol Rev. 2012. PMID: 22232371 Free PMC article. Review.
References
-
- Bangsborg J M, Shand G, Høiby N. Antibody response to major cross-reactive Legionella antigens during infection. In: Barbaree J M, Breiman R F, Dufour H P, editors. Legionella—current status and emergent prospectives. Washington, D.C.: American Society for Microbiology; 1993. pp. 26–29.
-
- Bass J W, Vincent J M, Person D A. The expanding spectrum of Bartonella infections. II. Cat-scratch disease. Pediatr Infect Dis J. 1997;16:163–179. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

