Fluorescence-based quantitative methods for detecting human immunodeficiency virus type 1-induced syncytia
- PMID: 10921977
- PMCID: PMC87184
- DOI: 10.1128/JCM.38.8.3055-3060.2000
Fluorescence-based quantitative methods for detecting human immunodeficiency virus type 1-induced syncytia
Abstract
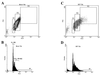
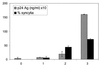
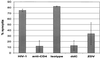
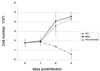
Cell fusion induced by human immunodeficiency virus type 1 (HIV-1) is usually assessed by counting multinucleated giant cells (syncytia) visualized by light microscopy. Currently used methods do not allow quantification of syncytia, nor do they estimate the number of cells involved in cell fusion. We describe two fluorescence-based methods for the detection and quantification of HIV-1-induced in vitro syncytium formation. The lymphoblastoid cell lines MT-2 and SupT1 were infected with syncytium-inducing (SI) HIV-1 isolates. Syncytia were detected by DNA staining with propidium iodide using flow cytometry to determine cell size or by two-color cytoplasmic staining of infected cell populations by using fluorescence microscopy. Both methods were able to detect and quantify HIV-induced syncytia. The methods could distinguish between SI and non-SI HIV isolates and could be used with at least two separate types of CD4(+) T-cell lines. Small syncytia can be readily identified by the two-color cytoplasmic staining method. Both methods were also shown to be useful for evaluating antiretroviral compounds, as demonstrated by the accurate assessment of HIV inhibition by azidothymidine (zidovudine), dideoxycytidine (zalcytibine), and hydroxyurea. These fluorescence-based assays allow a rapid and practical method for measuring HIV replication and anti-HIV activity of potential inhibitory compounds.
Figures


Similar articles
-
Syncytium induction in primary CD4+ T-cell lines from normal donors by human immunodeficiency virus type 1 isolates with non-syncytium-inducing genotype and phenotype in MT-2 cells.J Virol. 1995 Nov;69(11):7099-105. doi: 10.1128/JVI.69.11.7099-7105.1995. J Virol. 1995. PMID: 7474129 Free PMC article.
-
Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity.J Virol. 1992 Feb;66(2):757-65. doi: 10.1128/JVI.66.2.757-765.1992. J Virol. 1992. PMID: 1731110 Free PMC article.
-
Noninfectious doughnut-shaped human immunodeficiency virus type 1 can induce syncytia mediated by fusion of the particles with CD4-positive cells.J Acquir Immune Defic Syndr (1988). 1991;4(12):1233-40. J Acquir Immune Defic Syndr (1988). 1991. PMID: 1682474
-
HIV-envelope-dependent cell-cell fusion: quantitative studies.ScientificWorldJournal. 2009 Aug 11;9:746-63. doi: 10.1100/tsw.2009.90. ScientificWorldJournal. 2009. PMID: 19705036 Free PMC article. Review.
-
Syncytia in Fungi.Cells. 2020 Oct 8;9(10):2255. doi: 10.3390/cells9102255. Cells. 2020. PMID: 33050028 Free PMC article. Review.
Cited by
-
Wear particles derived from metal hip implants induce the generation of multinucleated giant cells in a 3-dimensional peripheral tissue-equivalent model.PLoS One. 2015 Apr 20;10(4):e0124389. doi: 10.1371/journal.pone.0124389. eCollection 2015. PLoS One. 2015. PMID: 25894745 Free PMC article.
-
Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor.J Virol. 2000 Nov;74(21):10055-62. doi: 10.1128/jvi.74.21.10055-10062.2000. J Virol. 2000. PMID: 11024134 Free PMC article.
-
GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates.J Immunol. 2010 Oct 1;185(7):4496-505. doi: 10.4049/jimmunol.1001980. Epub 2010 Sep 8. J Immunol. 2010. PMID: 20826757 Free PMC article.
-
Advances in biosensing strategies for HIV-1 detection, diagnosis, and therapeutic monitoring.Adv Drug Deliv Rev. 2016 Aug 1;103:90-104. doi: 10.1016/j.addr.2016.05.018. Epub 2016 Jun 2. Adv Drug Deliv Rev. 2016. PMID: 27262924 Free PMC article. Review.
-
Characterization of a peptide domain within the GB virus C NS5A phosphoprotein that inhibits HIV replication.PLoS One. 2008 Jul 2;3(7):e2580. doi: 10.1371/journal.pone.0002580. PLoS One. 2008. PMID: 18596910 Free PMC article.
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Anonymous. Immunoflourescence and cell sorting: use of flow cytometry for DNA analysis. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N. Y: John Wiley and Sons; 1991. pp. 5.7–5.7.2.
-
- Åsjö B, Morfeldt-Manson L, Albert J, Biberfeld G, Karlsson A, Lidman K, Fenyö E M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;ii:660–662. - PubMed
-
- Benyoucef S, Hober D, Shen L, Ajana F, Gérard Y, Bocket-Mouton L, Mouton Y, Wattré P. A microassay for determination of the cytopathogenicity of human immunodeficiency virus type-1 isolates. Microbiol Immunol. 1996;40:381–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

