Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I
- PMID: 10922006
- PMCID: PMC2270032
- DOI: 10.1111/j.1469-7793.2000.t01-1-00541.x
Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I
Abstract
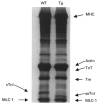
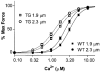
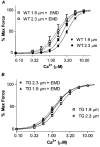
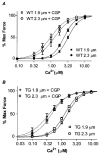
We compared sarcomere length (SL) dependence of the Ca2+-force relation of detergent-extracted bundles of fibres dissected from the left ventricle of wild-type (WT) and transgenic mouse hearts expressing slow skeletal troponin I (ssTnI-TG). Fibre bundles from the hearts of the ssTnI-TG demonstrated a complete replacement of the cardiac troponin I (cTnI) by ssTnI. Compared to WT controls, ssTnI-TG fibre bundles were more sensitive to Ca2+ at both short SL (1.9 +/- 0.1 micrometer) and long SL (2.3 +/- 0.1 micrometer). However, compared to WT controls, the increase in Ca2+ sensitivity (change in half-maximally activating free Ca2+; DeltaEC50) associated with the increase in SL was significantly blunted in the ssTnI-TG myofilaments. Agents that sensitize the myofilaments to Ca2+ by promoting the actin-myosin reaction (EMD 57033 and CGP-48506) significantly reduced the length-dependent DeltaEC50 for Ca2+ activation, when SL in WT myofilaments was increased from 1.9 to 2.3 micrometer. Exposure of myofilaments to calmidazolium (CDZ), which binds to cTnC and increases its affinity for Ca2+, sensitized force developed by WT myofilaments to Ca2+ at SL 1.9 micrometer and desensitized the WT myofilaments at SL 2.3 micrometer. There were no significant effects of CDZ on ssTnI-TG myofilaments at either SL. Our results indicate that length-dependent Ca2+ activation is modified by specific changes in thin filament proteins and by agents that promote the actin-myosin interaction. Thus, these in vitro results provide a basis for using these models to test the relative significance of the length dependence of activation in situ.
Figures

References
-
- Akella AB, Ding XL, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circulation Research. 1995;76:600–606. - PubMed
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. Journal of Molecular and Cellular Cardiology. 1985;17:821–840. - PubMed
-
- Dong W-J, Chandra M, Xing J, She M, Solaro RJ, Cheung HC. Phosphorylation-induced distance change in a cardiac muscle troponin I mutant. Biochemistry. 1997;36:6754–6761. - PubMed
-
- El-Saleh SC, Solaro RJ. Calmidazolium, a calmodulin antagonist, stimulates calcium-troponin C and calcium-calmodulin-dependent activation of striated muscle myofilaments. Journal of Biological Chemistry. 1987;262:17240–17246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

