Structure and function of pectic enzymes: virulence factors of plant pathogens
- PMID: 10922032
- PMCID: PMC34009
- DOI: 10.1073/pnas.97.16.8762
Structure and function of pectic enzymes: virulence factors of plant pathogens
Abstract
The structure and function of Erwinia chrysanthemi pectate lysase C, a plant virulence factor, is reviewed to illustrate one mechanism of pathogenesis at the molecular level. Current investigative topics are discussed in this paper.
Figures
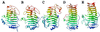
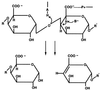
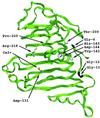

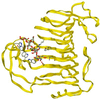

Similar articles
-
An exo-poly-alpha-D-galacturonosidase implicated in the regulation of extracellular pectate lyase production in Erwinia chrysanthemi.J Bacteriol. 1982 Feb;149(2):626-34. doi: 10.1128/jb.149.2.626-634.1982. J Bacteriol. 1982. PMID: 7056698 Free PMC article.
-
Comparison of pectic enzymes produced by Erwinia chrysanthemi, Erwinia carotovora subsp. carotovora, and Erwinia carotovora subsp. atroseptica.Appl Environ Microbiol. 1986 Aug;52(2):305-10. doi: 10.1128/aem.52.2.305-310.1986. Appl Environ Microbiol. 1986. PMID: 3752996 Free PMC article.
-
Enzymatic deconstruction of backbone structures of the ramified regions in pectins.Protein J. 2008 Jan;27(1):30-42. doi: 10.1007/s10930-007-9105-0. Protein J. 2008. PMID: 17823855 Review.
-
Preliminary crystallographic analysis of the plant pathogenic factor, pectate lyase C from Erwinia chrysanthemi.J Biol Chem. 1990 Jul 15;265(20):11429-31. J Biol Chem. 1990. PMID: 2195018
-
Pectic enzymes.Adv Carbohydr Chem Biochem. 1976;33:323-85. doi: 10.1016/s0065-2318(08)60285-1. Adv Carbohydr Chem Biochem. 1976. PMID: 11647 Review. No abstract available.
Cited by
-
Identification of amino acid residues critical for catalysis and stability in Aspergillus niger family 1 pectin lyase A.Biochem J. 2003 Feb 15;370(Pt 1):331-7. doi: 10.1042/BJ20021071. Biochem J. 2003. PMID: 12418964 Free PMC article.
-
Characterization of Major Cell-Wall-Degrading Enzymes Secreted by Diaporthe spp. Isolate Z1-1N Causing Postharvest Fruit Rot in Kiwifruit in China.Biology (Basel). 2024 Dec 2;13(12):1006. doi: 10.3390/biology13121006. Biology (Basel). 2024. PMID: 39765673 Free PMC article.
-
Cloning, expression and characterization of a metagenome derived thermoactive/thermostable pectinase.Mol Biol Rep. 2012 Aug;39(8):8353-61. doi: 10.1007/s11033-012-1685-x. Epub 2012 Jun 19. Mol Biol Rep. 2012. PMID: 22711301
-
Cloning of the Trichoderma reesei cDNA encoding a glucuronan lyase belonging to a novel polysaccharide lyase family.Appl Environ Microbiol. 2009 Jan;75(1):101-7. doi: 10.1128/AEM.01749-08. Epub 2008 Oct 31. Appl Environ Microbiol. 2009. PMID: 18978091 Free PMC article.
-
Study of the mode of action of a polygalacturonase from the phytopathogen Burkholderia cepacia.Biochem J. 2007 Oct 15;407(2):207-17. doi: 10.1042/BJ20061833. Biochem J. 2007. PMID: 17627609 Free PMC article.
References
-
- Carpita N C, Gibeaut D M. Plant J. 1993;3:1–30. - PubMed
-
- Albersheim P, Darvill A, O'Neill M, Schols H A, Voragen A G J. In: Progress in Biotechnology: Pectins and Pectinases. Visser J, Voragen A G J, editors. Vol. 14. Amsterdam: Elsevier; 1996. pp. 47–53.
-
- Davies G, Henrissat B. Structure (London) 1995;3:853–859. - PubMed
-
- Kiss J. Adv Carbohydr Chem Biochem. 1974;29:229–230.
-
- He S Y, Lindeberg M, Collmer A. In: Biotechnology in Plant Disease Control. Chet I, editor. New York: Wiley–Liss; 1993. pp. 39–64.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

