Exploitation of host cells by enteropathogenic Escherichia coli
- PMID: 10922038
- PMCID: PMC34015
- DOI: 10.1073/pnas.97.16.8799
Exploitation of host cells by enteropathogenic Escherichia coli
Abstract
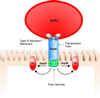
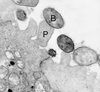
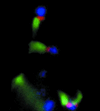
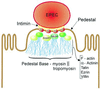
Microbial pathogens have evolved many ingenious ways to infect their hosts and cause disease, including the subversion and exploitation of target host cells. One such subversive microbe is enteropathogenic Escherichia coli (EPEC). A major cause of infantile diarrhea in developing countries, EPEC poses a significant health threat to children worldwide. Central to EPEC-mediated disease is its colonization of the intestinal epithelium. After initial adherence, EPEC causes the localized effacement of microvilli and intimately attaches to the host cell surface, forming characteristic attaching and effacing (A/E) lesions. Considered the prototype for a family of A/E lesion-causing bacteria, recent in vitro studies of EPEC have revolutionized our understanding of how these pathogens infect their hosts and cause disease. Intimate attachment requires the type III-mediated secretion of bacterial proteins, several of which are translocated directly into the infected cell, including the bacteria's own receptor (Tir). Binding to this membrane-bound, pathogen-derived protein permits EPEC to intimately attach to mammalian cells. The translocated EPEC proteins also activate signaling pathways within the underlying cell, causing the reorganization of the host actin cytoskeleton and the formation of pedestal-like structures beneath the adherent bacteria. This review explores what is known about EPEC's subversion of mammalian cell functions and how this knowledge has provided novel insights into bacterial pathogenesis and microbe-host interactions. Future studies of A/E pathogens in animal models should provide further insights into how EPEC exploits not only epithelial cells but other host cells, including those of the immune system, to cause diarrheal disease.
Figures
Similar articles
-
Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside.Cell Microbiol. 2000 Feb;2(1):1-9. doi: 10.1046/j.1462-5822.2000.00033.x. Cell Microbiol. 2000. PMID: 11207558 Review.
-
Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells.Cell Mol Life Sci. 1999 Jun;55(6-7):961-76. doi: 10.1007/pl00013202. Cell Mol Life Sci. 1999. PMID: 10412374 Free PMC article. Review.
-
Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated.Infect Immun. 1999 May;67(5):2389-98. doi: 10.1128/IAI.67.5.2389-2398.1999. Infect Immun. 1999. PMID: 10225900 Free PMC article.
-
EspFu-Mediated Actin Assembly Enhances Enteropathogenic Escherichia coli Adherence and Activates Host Cell Inflammatory Signaling Pathways.mBio. 2020 Apr 14;11(2):e00617-20. doi: 10.1128/mBio.00617-20. mBio. 2020. PMID: 32291304 Free PMC article.
-
Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells.Nat Cell Biol. 2001 Sep;3(9):856-9. doi: 10.1038/ncb0901-856. Nat Cell Biol. 2001. PMID: 11533668
Cited by
-
Initial adherence of EPEC, EHEC and VTEC to host cells.Vet Res. 2010 Sep-Oct;41(5):57. doi: 10.1051/vetres/2010029. Epub 2010 Apr 29. Vet Res. 2010. PMID: 20423697 Free PMC article. Review.
-
Conformation of the EPEC Tir protein in solution: investigating the impact of serine phosphorylation at positions 434/463.Biophys J. 2007 Jul 15;93(2):586-96. doi: 10.1529/biophysj.106.101766. Epub 2007 Apr 20. Biophys J. 2007. PMID: 17449672 Free PMC article.
-
Towards a physiology of epithelial pathogens.Pflugers Arch. 2002 Jan;443(3):339-43. doi: 10.1007/s00424-001-0729-1. Epub 2001 Nov 1. Pflugers Arch. 2002. PMID: 11810201 Review. No abstract available.
-
Ileal lesions in patients with ulcerative colitis after ileo-rectal anastomosis: relationship with colonic metaplasia.World J Gastroenterol. 2008 Sep 14;14(34):5290-300. doi: 10.3748/wjg.14.5290. World J Gastroenterol. 2008. PMID: 18785281 Free PMC article.
-
Molecular characterization of Escherichia coli O157:H7 recovered from meat and meat products relevant to human health in Riyadh, Saudi Arabia.Saudi J Biol Sci. 2015 Nov;22(6):725-9. doi: 10.1016/j.sjbs.2015.06.009. Epub 2015 Jun 17. Saudi J Biol Sci. 2015. PMID: 26587000 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

