The role of antimicrobial peptides in animal defenses
- PMID: 10922046
- PMCID: PMC34023
- DOI: 10.1073/pnas.97.16.8856
The role of antimicrobial peptides in animal defenses
Abstract
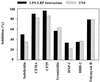
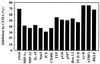
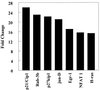
It is becoming clear that the cationic antimicrobial peptides are an important component of the innate defenses of all species of life. Such peptides can be constitutively expressed or induced by bacteria or their products. The best peptides have good activities vs. a broad range of bacterial strains, including antibiotic-resistant isolates. They kill very rapidly, do not easily select resistant mutants, are synergistic with conventional antibiotics, other peptides, and lysozyme, and are able to kill bacteria in animal models. It is known that bacterial infections, especially when treated with antibiotics, can lead to the release of bacterial products such as lipopolysaccharide (LPS) and lipoteichoic acid, resulting in potentially lethal sepsis. In contrast to antibiotics, the peptides actually prevent cytokine induction by bacterial products in tissue culture and human blood, and they block the onset of sepsis in mouse models of endotoxemia. Consistent with this, transcriptional gene array experiments using a macrophage cell line demonstrated that a model peptide, CEMA, blocks the expression of many genes whose transcription was induced by LPS. The peptides do this in part by blocking LPS interaction with the serum protein LBP. In addition, CEMA itself has a direct effect on macrophage gene expression. Because cationic antimicrobial peptides are induced by LPS and are able to dampen the septic response of animal cells to LPS, we propose that, in addition to their role in direct and lysozyme-assisted killing of microbes, they have a role in feedback regulation of cytokine responses. We are currently developing variant peptides as therapeutics against antibiotic-resistant infections.
Figures

Similar articles
-
Cationic antimicrobial peptides and their multifunctional role in the immune system.Crit Rev Immunol. 2000;20(5):407-31. Crit Rev Immunol. 2000. PMID: 11145218 Review.
-
Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria.Infect Immun. 1999 Dec;67(12):6445-53. doi: 10.1128/IAI.67.12.6445-6453.1999. Infect Immun. 1999. PMID: 10569762 Free PMC article.
-
Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis.Biochim Biophys Acta. 2016 May;1858(5):971-9. doi: 10.1016/j.bbamem.2016.01.011. Epub 2016 Jan 20. Biochim Biophys Acta. 2016. PMID: 26801369 Review.
-
Endotoxin-binding synthetic peptides with endotoxin-neutralizing, antibacterial and anticoagulant activities.Prog Clin Biol Res. 1994;388:147-59. Prog Clin Biol Res. 1994. PMID: 7831355
-
Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification.Antimicrob Agents Chemother. 1994 Oct;38(10):2311-6. doi: 10.1128/AAC.38.10.2311. Antimicrob Agents Chemother. 1994. PMID: 7840562 Free PMC article.
Cited by
-
Membrane-active peptides from marine organisms--antimicrobials, cell-penetrating peptides and peptide toxins: applications and prospects.Probiotics Antimicrob Proteins. 2015 Mar;7(1):75-89. doi: 10.1007/s12602-014-9182-2. Probiotics Antimicrob Proteins. 2015. PMID: 25559972 Review.
-
Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis Virulence: Implication for Skin Homeostasis.Front Microbiol. 2016 Apr 15;7:506. doi: 10.3389/fmicb.2016.00506. eCollection 2016. Front Microbiol. 2016. PMID: 27148195 Free PMC article.
-
Treatment and prevention of Staphylococcus epidermidis experimental biomaterial-associated infection by bactericidal peptide 2.Antimicrob Agents Chemother. 2006 Dec;50(12):3977-83. doi: 10.1128/AAC.00575-06. Epub 2006 Sep 25. Antimicrob Agents Chemother. 2006. PMID: 17000746 Free PMC article.
-
The Bacillus subtilis extracytoplasmic-function sigmaX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides.J Bacteriol. 2004 Feb;186(4):1136-46. doi: 10.1128/JB.186.4.1136-1146.2004. J Bacteriol. 2004. PMID: 14762009 Free PMC article.
-
Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3587-90. doi: 10.1073/pnas.0308750101. Epub 2004 Feb 17. Proc Natl Acad Sci U S A. 2004. PMID: 14970327 Free PMC article.
References
-
- Hancock R E W, Lehrer R. Trends Biotechnol. 1998;16:82–88. - PubMed
-
- Hetru C, Hoffmann D, Bulet P. In: Molecular Mechanisms of Immune Responses in Insects. Brey P T, Hultmark D, editors. London: Chapman & Hall; 1998. pp. 40–66.
-
- Schroder J-M, Jurgen H. Int J Biochem Cell Biol. 1999;31:645–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

