Suramin inhibits initiation of defense signaling by systemin, chitosan, and a beta-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells
- PMID: 10922047
- PMCID: PMC34024
- DOI: 10.1073/pnas.97.16.8862
Suramin inhibits initiation of defense signaling by systemin, chitosan, and a beta-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells
Abstract
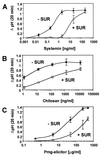
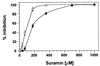
Systemin-mediated defense signaling in tomato (Lycopersicon esculentum) plants is analogous to the cytokine-mediated inflammatory response in animals. Herein, we report that the initiation of defense signaling in suspension-cultured cells of Lycopersicon peruvianum by the peptide systemin, as well as by chitosan and beta-glucan elicitor from Phytophtora megasperma, is inhibited by the polysulfonated naphtylurea compound suramin, a known inhibitor of cytokine and growth factor receptor interactions in animal cells. Using a radioreceptor assay, we show that suramin interfered with the binding of the systemin analog (125)I-Tyr-2, Ala-15-systemin to the systemin receptor with an IC(50) of 160 microM. Additionally, labeling of the systemin receptor with a photoaffinity analog of systemin was inhibited in the presence of suramin. Receptor-mediated tyrosine phosphorylation of a 48-kDa mitogen-activated protein kinase and alkalinization of the medium of suspension-cultured cells in response to systemin and carbohydrate elicitors were also inhibited by suramin. The inhibition of medium alkalinization by suramin was reversible in the presence of high concentrations of systemin and carbohydrate elicitors. Calyculin A and erythrosin B, intracellular inhibitors of phosphatases and plasma membrane proton ATPases, respectively, both induce medium alkalinization, but neither response was inhibited by suramin. The polysulfonated compound heparin did not inhibit systemin-induced medium alkalinization. NF 007, a suramin derivative, induced medium alkalinization, indicating that neither NF 007 nor heparin interact with elicitor receptors like suramin. The data indicate that cell-surface receptors in plants show some common structural features with animal cytokine and growth factor receptors that can interact with suramin to interfere with ligand binding.
Figures





Similar articles
-
Ultraviolet-B activates components of the systemin signaling pathway in Lycopersicon peruvianum suspension-cultured cells.J Biol Chem. 2002 Aug 9;277(32):28424-30. doi: 10.1074/jbc.M203844200. Epub 2002 May 28. J Biol Chem. 2002. PMID: 12034744
-
A 160-kD systemin receptor on the surface of lycopersicon peruvianum suspension-cultured cells.Plant Cell. 1999 Aug;11(8):1525-36. doi: 10.1105/tpc.11.8.1525. Plant Cell. 1999. PMID: 10449585 Free PMC article.
-
Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants.Plant Cell. 1999 Feb;11(2):263-72. doi: 10.1105/tpc.11.2.263. Plant Cell. 1999. PMID: 9927643 Free PMC article.
-
Systemin, hydroxyproline-rich systemin and the induction of protease inhibitors.Curr Protein Pept Sci. 2011 Aug;12(5):399-408. doi: 10.2174/138920311796391106. Curr Protein Pept Sci. 2011. PMID: 21418016 Review.
-
Systemin/Jasmonate-mediated systemic defense signaling in tomato.Mol Plant. 2011 Jul;4(4):607-15. doi: 10.1093/mp/ssr008. Epub 2011 Feb 28. Mol Plant. 2011. PMID: 21357647 Review.
Cited by
-
Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora.Plant Physiol. 2003 Aug;132(4):1755-67. doi: 10.1104/pp.103.024323. Plant Physiol. 2003. PMID: 12913133 Free PMC article.
-
Systemins: a functionally defined family of peptide signals that regulate defensive genes in Solanaceae species.Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2(Suppl 2):14577-80. doi: 10.1073/pnas.1934788100. Epub 2003 Aug 29. Proc Natl Acad Sci U S A. 2003. PMID: 12949264 Free PMC article.
-
UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures.BMC Plant Biol. 2007 Nov 7;7:61. doi: 10.1186/1471-2229-7-61. BMC Plant Biol. 2007. PMID: 17988378 Free PMC article.
-
Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells.Plant Physiol. 2003 Aug;132(4):1728-38. doi: 10.1104/pp.103.024414. Plant Physiol. 2003. PMID: 12913131 Free PMC article.
-
LeRALF, a plant peptide that regulates root growth and development, specifically binds to 25 and 120 kDa cell surface membrane proteins of Lycopersicon peruvianum.Planta. 2005 Jul;221(5):667-74. doi: 10.1007/s00425-004-1442-z. Epub 2005 May 21. Planta. 2005. PMID: 15909150
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

