The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex
- PMID: 10922056
- PMCID: PMC16806
- DOI: 10.1073/pnas.97.16.8973
The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex
Abstract
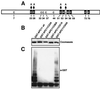
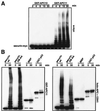
The anaphase-promoting complex (APC) is a cell cycle-regulated ubiquitin-protein ligase that targets cyclin B, securin and other destruction box containing proteins for proteolysis. Nine APC subunits have been identified in vertebrates and eleven in yeast, but for none of them it is known how they contribute to the catalysis of ubiquitination reactions. Here we report the mass spectrometric identification of CDC26 and of the RING-H2 finger protein APC11 in the human APC. We have expressed these proteins and several other APC subunits in Escherichia coli and have tested their activities in vitro. We find that APC11 alone is sufficient to allow the synthesis of multiubiquitin chains in the presence of E1 and UBC4. These multiubiquitin chains are partly unanchored and partly bound to APC11 itself. APC11 and UBC4 are also able to ubiquitinate securin and cyclin B, but these reactions show a decreased dependency on the destruction box. The integrity of the putative zinc binding RING-H2 finger is required for the ability of APC11 to support ubiquitination reactions. These results suggest that APC11 and UBC4 catalyze the formation of isopeptide bonds in APC-mediated ubiquitination reactions.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

