Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo
- PMID: 10922064
- PMCID: PMC16827
- DOI: 10.1073/pnas.97.16.9093
Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo
Abstract
The dorsoventral axis of the Drosophila embryo is induced by a ventrally restricted ligand for the receptor Toll. The Toll ligand is generated by a proteolytic processing reaction, which occurs at the end of a proteolytic cascade and requires the gastrulation defective (gd), nudel, pipe, and windbeutel genes. Here we demonstrate that the GD protein is a serine protease and that the three other genes act to restrict GD activity to the ventral side of the embryo. Our data support a model in which the GD protease catalyzes the ventral activation of the proteolytic cascade that produces the Toll ligand.
Figures

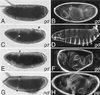
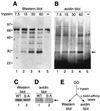
Similar articles
-
A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity.Curr Biol. 2012 Jun 5;22(11):1013-8. doi: 10.1016/j.cub.2012.03.065. Epub 2012 May 10. Curr Biol. 2012. PMID: 22578419 Free PMC article.
-
Localized serine protease activity and the establishment of Drosophila embryonic dorsoventral polarity.Fly (Austin). 2013 Jul-Sep;7(3):161-7. doi: 10.4161/fly.25141. Epub 2013 Jun 10. Fly (Austin). 2013. PMID: 24047959 Free PMC article.
-
Signal transduction by a protease cascade.Trends Cell Biol. 1999 Mar;9(3):102-7. doi: 10.1016/s0962-8924(98)01494-9. Trends Cell Biol. 1999. PMID: 10201075 Review.
-
Regulation of translation and proteolysis during the development of embryonic dorso-ventral polarity in Drosophila. Homology of easter proteinase with Limulus proclotting enzyme and translational activation of Toll receptor synthesis.Biochim Biophys Acta. 1992 Oct 20;1132(3):290-6. doi: 10.1016/0167-4781(92)90163-t. Biochim Biophys Acta. 1992. PMID: 1420309
-
Axis determination. Proteolytic generation of a morphogen.Curr Biol. 1994 Aug 1;4(8):755-7. doi: 10.1016/s0960-9822(00)00170-6. Curr Biol. 1994. PMID: 7953570 Review.
Cited by
-
Profiling ATM regulated genes in Drosophila at physiological condition and after ionizing radiation.Hereditas. 2022 Oct 21;159(1):41. doi: 10.1186/s41065-022-00254-9. Hereditas. 2022. PMID: 36271387 Free PMC article.
-
Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila.PLoS One. 2013 Apr 24;8(4):e61773. doi: 10.1371/journal.pone.0061773. Print 2013. PLoS One. 2013. PMID: 23637905 Free PMC article.
-
Three-dimensional morphology and gene expression in the Drosophila blastoderm at cellular resolution II: dynamics.Genome Biol. 2006;7(12):R124. doi: 10.1186/gb-2006-7-12-r124. Genome Biol. 2006. PMID: 17184547 Free PMC article.
-
Spatially Restricted Regulation of Spätzle/Toll Signaling during Cell Competition.Dev Cell. 2018 Sep 24;46(6):706-719.e5. doi: 10.1016/j.devcel.2018.08.001. Epub 2018 Aug 23. Dev Cell. 2018. PMID: 30146479 Free PMC article.
-
No requirement for localized Nudel protein expression in Drosophila embryonic axis determination.Fly (Austin). 2008 Jul-Aug;2(4):220-8. doi: 10.4161/fly.6794. Fly (Austin). 2008. PMID: 18776742 Free PMC article.
References
-
- Morisato D, Anderson K V. Annu Rev Genet. 1995;29:371–399. - PubMed
-
- Morisato D, Anderson K V. Cell. 1994;76:677–688. - PubMed
-
- Schneider D S, Jin Y, Morisato D, Anderson K V. Development (Cambridge, UK) 1994;120:1243–1250. - PubMed
-
- DeLotto R, Spierer P. Nature (London) 1986;323:688–692. - PubMed
-
- Chasan R, Anderson K V. Cell. 1989;56:391–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

