3' poly(A) is dispensable for translation
- PMID: 10922069
- PMCID: PMC16834
- DOI: 10.1073/pnas.97.16.9133
3' poly(A) is dispensable for translation
Abstract
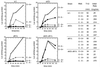
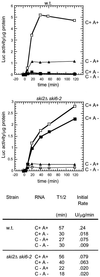
In wild-type cells, the 3' poly(A) structure is necessary for translation of mRNA and for mRNA stability. The superkiller 2 (ski2), ski3, ski6, ski7, and ski8 mutations enhance the expression of the poly(A)(-) mRNAs of yeast RNA viruses. Ski2p is a DEVH-box RNA helicase and Slh1p resembles Ski2p. Both repress L-A double-stranded RNA (dsRNA) virus copy number, further suggesting that their functions may overlap. We find that slh1Delta ski2Delta double mutants are healthy (in the absence of viruses) and show normal rates of turnover of several cellular mRNAs. The slh1Delta ski2Delta strains translate electroporated nonpoly(A) mRNA with the same kinetics as polyA(+) mRNA. Thus, the translation apparatus is inherently capable of efficiently using nonpoly(A) mRNA even in the presence of normal amounts of competing poly(A)(+) mRNA, but is normally prevented from doing so by the combined action of the nonessential proteins Ski2p and Slh1p.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

