Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits
- PMID: 10922076
- PMCID: PMC16856
- DOI: 10.1073/pnas.97.16.9264
Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits
Abstract
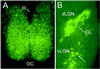
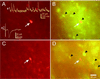
Physiological properties of central nervous system neurons infected with a pseudorabies virus were examined in vitro by using whole-cell patch-clamp techniques. A strain of pseudorabies virus (PRV 152) isogenic with the Bartha strain of PRV was constructed to express an enhanced green fluorescent protein (EGFP) from the human cytomegalovirus immediate early promoter. Unilateral PRV 152 injections into the vitreous body of the hamster eye transsynaptically infected a restricted set of retinorecipient neurons including neurons in the hypothalamic suprachiasmatic nucleus (SCN) and the intergeniculate leaflet (IGL) of the thalamus. Retinorecipient SCN neurons were identified in tissue slices prepared for in vitro electrophysiological analysis by their expression of EGFP. At longer postinjection times, retinal ganglion cells in the contralateral eye also expressed EGFP, becoming infected after transsynaptic uptake and retrograde transport from infected retinorecipient neurons. Retinal ganglion cells that expressed EGFP were easily identified in retinal whole mounts viewed under epifluorescence. Whole-cell patch-clamp recordings revealed that the physiological properties of PRV 152-infected SCN neurons were within the range of properties observed in noninfected SCN neurons. Physiological properties of retinal ganglion cells also appeared normal. The results suggest that PRV 152 is a powerful tool for the transsynaptic labeling of neurons in defined central nervous system circuits that allows neurons to be identified in vitro by their expression of EGFP, analyzed electrophysiologically, and described in morphological detail.
Figures


References
-
- Card J P. Anat Rec. 1998;253:176–185. - PubMed
-
- Card J P, Enquist L W, Moore R Y. J Comp Neurol. 1999;407:438–452. - PubMed
-
- Chen S, Yang M, Miselis R R, Aston-Jones G. Brain Res. 1999;838:171–183. - PubMed
-
- Enquist L W, Husak P J, Banfield B W, Smith G A. Adv Virus Res. 1999;51:237–347. - PubMed
-
- Kuypers H G J M, Ugolini G. Trends Neurosci. 1990;13:71–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

