Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid beta-protein generation
- PMID: 10922078
- PMCID: PMC16862
- DOI: 10.1073/pnas.97.16.9299
Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid beta-protein generation
Abstract
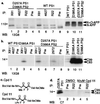
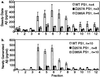
An unusual intramembranous cleavage of the beta-amyloid precursor protein (APP) by gamma-secretase is the final step in the generation of amyloid beta-peptide (Abeta). Two conserved aspartates in transmembrane (TM) domains 6 and 7 of presenilin (PS) 1 are required for Abeta production by gamma-secretase. Here we report that the APP C-terminal fragments, C83 and C99, which are the direct substrates of gamma-secretase, can be coimmunoprecipitated with both PS1 and PS2. PS/C83 complexes were detected in cells expressing endogenous levels of PS. The complexes accumulate when gamma-secretase is inactivated either pharmacologically or by mutating the PS aspartates. PS1/C83 and PS1/C99 complexes were detected in Golgi-rich and trans-Golgi network-rich vesicle fractions. In contrast, complexes of PS1 with APP holoprotein, which is not the immediate substrate of gamma-secretase, occurred earlier in endoplasmic reticulum-rich vesicles. The major portion of intracellular Abeta at steady state was found in the same Golgi/trans-Golgi network-rich vesicles, and Abeta levels in these fractions were markedly reduced when either PS1 TM aspartate was mutated to alanine. Furthermore, de novo generation of Abeta in a cell-free microsomal reaction occurred specifically in these same vesicle fractions and was markedly inhibited by mutating either TM aspartate. Thus, PSs are complexed with the gamma-secretase substrates C83 and C99 in the subcellular locations where Abeta is generated, indicating that PSs are directly involved in the pathogenically critical intramembranous proteolysis of APP.
Figures


Similar articles
-
The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation.J Biol Chem. 2000 Feb 4;275(5):3173-8. doi: 10.1074/jbc.275.5.3173. J Biol Chem. 2000. PMID: 10652302
-
Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412
-
Presenilin 1 stabilizes the C-terminal fragment of the amyloid precursor protein independently of gamma-secretase activity.J Biol Chem. 2004 Jun 11;279(24):25333-8. doi: 10.1074/jbc.M312710200. Epub 2004 Apr 15. J Biol Chem. 2004. PMID: 15087467
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
-
Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
Cited by
-
Potential treatment opportunities for Alzheimer's disease through inhibition of secretases and Abeta immunization.J Mol Neurosci. 2001 Oct;17(2):259-67. doi: 10.1385/JMN:17:2:259. J Mol Neurosci. 2001. PMID: 11816797 Review.
-
In vivo reconstitution of gamma-secretase in Drosophila results in substrate specificity.Mol Cell Biol. 2010 Jul;30(13):3165-75. doi: 10.1128/MCB.00030-10. Epub 2010 Apr 26. Mol Cell Biol. 2010. PMID: 20421416 Free PMC article.
-
Amyloid metabolism and secretases in Alzheimer's disease.Curr Neurol Neurosci Rep. 2001 Sep;1(5):422-7. doi: 10.1007/s11910-001-0101-z. Curr Neurol Neurosci Rep. 2001. PMID: 11898552 Review.
-
Calsyntenin-1 mediates axonal transport of the amyloid precursor protein and regulates Aβ production.Hum Mol Genet. 2012 Jul 1;21(13):2845-54. doi: 10.1093/hmg/dds109. Epub 2012 Mar 20. Hum Mol Genet. 2012. PMID: 22434822 Free PMC article.
-
Turnover of C99 is controlled by a crosstalk between ERAD and ubiquitin-independent lysosomal degradation in human neuroglioma cells.PLoS One. 2013 Dec 20;8(12):e83096. doi: 10.1371/journal.pone.0083096. eCollection 2013. PLoS One. 2013. PMID: 24376644 Free PMC article.
References
-
- Selkoe D J. Nature (London) 1999;399:A23–A31. - PubMed
-
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Gundula G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. - PubMed
-
- Xia W, Zhang J, Ostaszewski B, Kimberly W, Seubert P, Koo E, Selkoe D. Biochemistry. 1998;37:16465–16471. - PubMed
-
- Naruse S, Thinakaran G, Luo J, Kusiak J W, Tomita T, Iwatsubo T, Qian X, Ginty D D, Price D L, Borchelt D R, et al. Neuron. 1998;21:1213–1221. - PubMed
-
- Wolfe M S, Xia W, Ostaszewski B L, Diehl T S, Kimberly W T, Selkoe D J. Nature (London) 1999;398:513–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

