Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation
- PMID: 10922080
- PMCID: PMC16865
- DOI: 10.1073/pnas.97.16.9317
Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation
Abstract
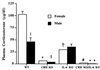
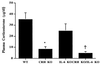
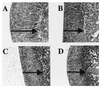
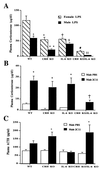
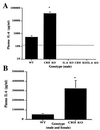
Glucocorticoids play a critical role in control of the cytokine response after immune challenge. Conversely, cytokines modulate glucocorticoid production by the hypothalamic-pituitary-adrenal axis. To define the potency and mechanism of interleukin-6 (IL-6) for augmentation of adrenal function, we exploited mice deficient in corticotropin-releasing hormone (CRH), IL-6, or both. Mice deficient in CRH action demonstrate severely impaired glucocorticoid production in response to psychological and metabolic challenge, but near normal responses to stressors that activate the immune system. In this paper, we demonstrate that IL-6 is essential for activation of the hypothalamic-pituitary-adrenal axis during immunological challenge in the absence of hypothalamic input from CRH. IL-6 receptors are present on pituitary corticotrophs and adrenocortical cells, consistent with the ability of IL-6 to bypass CRH in augmentation of adrenal function. Plasma corticosterone levels after bacterial lipopolysaccharide injection in mice deficient in CRH or IL-6 were significantly lower than in wild-type mice but significantly greater than in mice deficient in both CRH and IL-6. A second model of immune system activation using 2C11, an antibody to the T cell receptor, demonstrated a normal corticosterone response in mice deficient in CRH or IL-6, but a markedly decreased response in mice deficient in both CRH and IL-6. Surprisingly, the relative contribution of IL-6 for modulation of the adrenal response to stress is greater in female than in male mice. This gender-specific difference in IL-6 action in mice suggests the utility of further analysis of IL-6 in determining the female predominance seen in many human inflammatory/autoimmune diseases.
Figures


References
-
- Chrousos G P. N Engl J Med. 1995;332:1351–1362. - PubMed
-
- Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. - PubMed
-
- Givalois L, Dornand J, Mekaouche M, Solier M D, Bristow A F, Ixart G, Siaud P, Assenmacher I, Barbanel G. Am J Physiol. 1994;267:R164–R170. - PubMed
-
- Karalis K, Muglia L J, Bae D, Hilderbrand H, Majzoub J A. J Neuroimmunol. 1997;72:131–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

