Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety
- PMID: 10926587
- PMCID: PMC27447
- DOI: 10.1136/bmj.321.7257.329
Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety
Abstract
Objectives: To determine whether use of an oral nicotine inhaler can result in long term reduction in smoking and whether concomitant use of nicotine replacement and smoking is safe.
Design: Double blind, randomised, placebo controlled trial. Four month trial with a two year follow up.
Setting: Two university hospital pulmonary clinics in Switzerland.
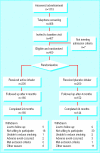
Participants: 400 healthy volunteers, recruited through newspaper advertisements, willing to reduce their smoking but unable or unwilling to stop smoking immediately.
Intervention: Active or placebo inhaler as needed for up to 18 months, with participants encouraged to limit their smoking as much as possible.
Main outcome measures: Number of cigarettes smoked per day from week six to end point. Decrease verified by a measurement of exhaled carbon monoxide at each time point compared with measurement at baseline.
Results: At four months sustained reduction of smoking was achieved in 52 (26%) participants in the active group and 18 (9%) in the placebo group (P<0.001; Fisher's test). Corresponding figures after two years were 19 (9.5%) and 6 (3.0%) (P=0.012).
Conclusion: Nicotine inhalers effectively and safely achieved sustained reduction in smoking over 24 months. Reduction with or without nicotine substitution may be a feasible first step towards smoking cessation in people not able or not willing to stop abruptly.
Figures
Comment in
-
Improving the treatment of tobacco dependence.BMJ. 2000 Aug 5;321(7257):311-2. doi: 10.1136/bmj.321.7257.311. BMJ. 2000. PMID: 10926568 Free PMC article. No abstract available.
References
-
- Smoking Cessation Clinical Practice Guideline Panel and Staff. The agency for health care policy and research: smoking cessation clinical practice guideline. JAMA. 1996;275:1270–1280. - PubMed
-
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Ann Rev Pharmacol Toxicol. 1996;36:597–613. - PubMed
-
- Silagy C, Mant D, Fowler G, Lancaster T Cochrane Collaboration, editors. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Nicotine replacement therapy for smoking cessation.
-
- Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9:643–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical