Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats
- PMID: 10928976
- PMCID: PMC1572233
- DOI: 10.1038/sj.bjp.0703479
Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats
Abstract
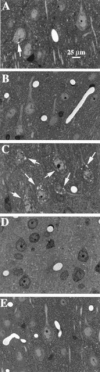
For general anaesthesia, patients usually receive a combination of drugs, all of which are classified as gamma-amino-butyric acid (GABA) agonists, with two notable exceptions - ketamine and nitrous oxide (laughing gas, N(2)O) - which are antagonists of N-methyl-D-aspartate (NMDA) glutamate receptors. At clinically relevant doses both ketamine and N(2)O, like other NMDA antagonists, have the potential to induce psychotomimetic reactions in humans and to cause pathomorphological changes in cerebrocortical neurons in rat brain. Because drug combinations used in clinical anaesthesia sometimes include both ketamine and N(2)O, we undertook experiments to evaluate whether augmented neurotoxicity results from their combined use. Ketamine and N(2)O were administered alone or in combination by various dosing regimens to adult female rats for a duration of 3 h and the severity of cerebrocortical neurotoxic changes was quantified histologically. Because GABA agonists are known to protect against the psychotomimetic and neurotoxic effects of NMDA antagonists, we also evaluated whether the combined neurotoxicity of ketamine+N(2)O can be prevented by certain commonly used GABA agonists. When ketamine and N(2)O were used in combination the neurotoxic reaction was enhanced to a degree much greater than can be explained by simple additivity. The apparent synergistic interaction was particularly striking when low doses of the agents were combined, the degree of toxic synergism at higher doses being masked by a ceiling effect. GABA agonists protected against ketamine/N(2)O neurotoxicity. It is recommended that this information be taken into consideration in the selection of drugs to be used in multi-agent protocols for general anaesthesia.
Figures




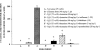
References
-
- BERGGREN D., GUSTAFSON Y., ERIKKSON B., BUCHT G., HANSSON L.I., REIZ S., WINBLAD B. Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anest. Analg. 1987;66:497. - PubMed
-
- BOVILL J.G., COPPELL L., DUNDEE J.W., MOORE J. Current status of ketamine anaesthesia. Lancet. 1971;1:1285. - PubMed
-
- DOHRN C.S., LICHTOR J.L., COALSON D.W., VITVLUGT A., DEWIT, ZACNY J.P. Reinforcing effects of extended inhalation of nitrous oxide in humans. Drug Alcohol Depend. 1993;31:265–280. - PubMed
-
- EDWARDS R.P., MILLER R.D., ROIZEN M.F., HAM J., WAY W.L., LAKE C.R., RODERICK L. Cardiac responses to imipramine and pancuronium during anesthesia with halothane or enflurane. Anesthesiology. 1979;50:421–425. - PubMed
-
- FIX A.S., HORN J.W., WIGHTMAN K.A., JOHNSON C.A., LONG G.G., STORTS R.W., FARBER N., WOZNIAK D.F., OLNEY J.W. Neuronal vacuolization and necrosis induced by the non-competitive N-methyl-D-aspartate (NMDA) antagonist MK (+)801 (Dizocilipine Maleate): A light and electron microscopic evaluation of the rat retrosplenial cortex. Exp. Neurol. 1993;123:204–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

