Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli
- PMID: 10929079
- PMCID: PMC2327037
- DOI: 10.1046/j.1365-2567.2000.00069.x
Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli
Abstract
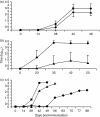
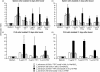
The delivery of antigens to mucosal-associated lymphoid tissues in paediatric and immunocompromised populations by safe, non-invasive vectors, such as commensal lactobacilli, represents a crucial improvement to prevailing vaccination options. In this report, we describe the oral and nasal immunization of mice with vaccines constructed through an original system for heterologous gene expression in Lactobacillus in which the 50 000-molecular weight (MW) fragment C of tetanus toxin (TTFC) is expressed either as an intracellular or a surface-exposed protein. Our data indicate that L. plantarum is more effective in this respect than L. casei and that, under the experimental conditions investigated, delivery of TTFC expressed as an intracellular antigen is more effective than cell-surface expression. Immunization of mice with live recombinant lactobacilli induced significant levels of circulating TTFC-specific immunoglobulin G (IgG) following nasal or oral delivery of vaccine strains. In addition, following nasal delivery, secretory immunoglobulin A (sIgA) was induced in bronchoalveolar lavage fluids, as were antigen-specific antibody-secreting cells and antigen-specific T-cell activation in draining lymph nodes, substantiating their potential for safe mucosal delivery of paediatric vaccines.
Figures


References
-
- Walker RI. New strategies for using mucosal vaccination to achieve more effective immunisation. Vaccine. 1994;12:387. - PubMed
-
- Curtiss R. Attenuated Salmonella strains as live vectors for the expression of foreign antigens. In: Woodrow G C, Levine M M, editors. New Generation Vaccines. New York: Marcel Dekker Inc; 1990. p. 347.
-
- Stover CK, de la Crus VF, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456. - PubMed
-
- Butterton JR, Boyko SA, Calderwood SB. Use of the Vibrio cholerae IgA gene as a locus for insertion and expression of heterologous antigens in cholera vaccine strains. Vaccine. 1993;11:1327. - PubMed
-
- Wells JM, Robinson K, Chamberlain LM, et al. Lactic acid bacteria as vaccine delivery vehicles. In: Venema G, Huis in't Veld JHJ, Hugenholtz J, editors. Lactic Acid Bacteria: Genetics, Metabolism and Applications. Dordrecht: Kluwer Academic Publishers; 1996. pp. 221–234.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

