Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy
- PMID: 10930440
- PMCID: PMC314323
- DOI: 10.1172/JCI8273
Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy
Erratum in
-
Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy.J Clin Invest. 2000 Sep;106(6):803. doi: 10.1172/jci8273c1. J Clin Invest. 2000. PMID: 10995791 Free PMC article. No abstract available.
Abstract
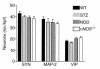
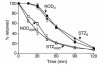
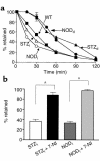
Gastrointestinal dysfunction is common in diabetic patients. In genetic (nonobese diabetic) and toxin-elicited (streptozotocin) models of diabetes in mice, we demonstrate defects in gastric emptying and nonadrenergic, noncholinergic relaxation of pyloric muscle, which resemble defects in mice harboring a deletion of the neuronal nitric oxide synthase gene (nNOS). The diabetic mice manifest pronounced reduction in pyloric nNOS protein and mRNA. The decline of nNOS in diabetic mice does not result from loss of myenteric neurons. nNOS expression and pyloric function are restored to normal levels by insulin treatment. Thus diabetic gastropathy in mice reflects an insulin-sensitive reversible loss of nNOS. In diabetic animals, delayed gastric emptying can be reversed with a phosphodiesterase inhibitor, sildenafil. These findings have implications for novel therapeutic approaches and may clarify the etiology of diabetic gastropathy.
Figures






References
-
- Porte, D., Jr., and Halter, J.B. 1999. The clinical syndrome of diabetes mellitus. In Diabetic neuropathy. P.J. Dyck and P.K. Thomas, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 1–28.
-
- Foster, D.W. 1998. Diabetes mellitus. In Harrison’s principles of internal medicine. A.S. Fauci et al., editors. McGraw-Hill. New York, New York, USA. 2060–2081.
-
- Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. - PubMed
-
- Abrahamsson H. Gastrointestinal motility disorders in patients with diabetes mellitus. J Intern Med. 1995;237:403–409. - PubMed
-
- Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

