Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen
- PMID: 10930441
- PMCID: PMC314330
- DOI: 10.1172/JCI9896
Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen
Abstract
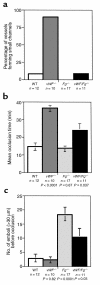
We used intravital microscopy to observe the formation of platelet plugs in ferric chloride-injured arterioles of live mice. With this model, we evaluated thrombus growth in mice lacking von Willebrand factor (vWF) and fibrinogen (Fg), the two key ligands known to mediate platelet adhesion and aggregation. In vWF(-/-) mice, despite the presence of arterial shear, delayed platelet adhesion occurred and stable thrombi formed. In many mice, a persisting high-shear channel never occluded. Abundant thrombi formed in Fg(-/-) mice, but they detached from the subendothelium, which ultimately caused downstream occlusion in all cases. Surprisingly, mice deficient in both vWF and Fg successfully formed thrombi with properties characteristic of both mutations, leading to vessel occlusion in the majority of vessels. Platelets of these doubly deficient mice specifically accumulated fibronectin in their alpha-granules, suggesting that fibronectin could be the ligand supporting the platelet aggregation.
Figures



References
-
- von Willebrand E. Hereditar psseudohemofili. Fin Lakaresallsk Handl. 1926;67:87–112.
-
- Gugler E, Lüscher EF. Platelet function in congenital afibrinogenemia. Thromb Diath Haemorrh. 1965;14:361–373. - PubMed
-
- Ewenstein BM. von Willebrand’s disease. Annu Rev Med. 1997;48:525–542. - PubMed
-
- Martinez J. Congenital dysfibrinogenemia. Curr Opin Hematol. 1997;4:357–365. - PubMed
-
- Tschopp TB, Weiss HJ, Baumgartner HR. Decreased adhesion of platelets to subendothelium in von Willebrand’s disease. J Lab Clin Med. 1974;83:296–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

