In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium
- PMID: 10930443
- PMCID: PMC314327
- DOI: 10.1172/JCI9560
In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium
Abstract
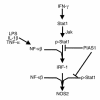
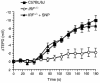
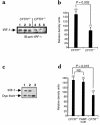
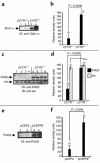
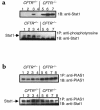
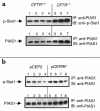
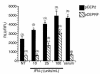
Inducible nitric oxide synthase-2 (NOS2) expression has been shown to be reduced in cystic fibrosis (CF) epithelial cells. Reduced NOS2 expression is unexpected, given the inflammatory nature of CF airway disease, and is an indication that cell-signaling mechanisms necessary for proper NOS2 regulation are probably altered in CF epithelium. Therefore, we examined the expression levels of regulatory factors necessary for NOS2 expression in CF epithelium and showed that IFN regulatory factor-1 (IRF-1) is necessary for full NOS2 expression. Mice lacking IRF-1 expression have diminished epithelial NOS2 expression, as well as reduced NO-dependent chloride transport across the nasal epithelia. Furthermore, IRF-1 protein expression is reduced in nasal and intestinal epithelial cells from CF mice, suggesting a possible mechanism for the CF-related reduction of epithelial NOS2 expression. Active signal transducer and activator of transcription-1 (Stat1) is necessary for both NOS2 and IRF-1 expression. We found that protein levels of Stat1 were increased in CF cells, but that the active phosphorylated form of Stat1 was bound to the protein inhibitor of activated Stat1 (PIAS1). We propose that increased levels of PIAS1 diminish certain cell-signaling pathways, resulting in reduced IRF-1 and NOS2 expression in CF epithelial cells.
Figures

Similar articles
-
Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2003 Dec;285(6):L1286-95. doi: 10.1152/ajplung.00127.2003. Epub 2003 Aug 29. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 12948935
-
Reduced Smad3 protein expression and altered transforming growth factor-beta1-mediated signaling in cystic fibrosis epithelial cells.Am J Respir Cell Mol Biol. 2001 Dec;25(6):732-8. doi: 10.1165/ajrcmb.25.6.4574. Am J Respir Cell Mol Biol. 2001. PMID: 11726399
-
Double-stranded rna dependence of nitric oxide synthase 2 expression in human bronchial epithelial cell lines BET-1A and BEAS-2B.Am J Respir Cell Mol Biol. 2001 Jun;24(6):720-6. doi: 10.1165/ajrcmb.24.6.4297. Am J Respir Cell Mol Biol. 2001. PMID: 11415937
-
16-kDa prolactin down-regulates inducible nitric oxide synthase expression through inhibition of the signal transducer and activator of transcription 1/IFN regulatory factor-1 pathway.Cancer Res. 2005 Sep 1;65(17):7984-92. doi: 10.1158/0008-5472.CAN-05-0631. Cancer Res. 2005. PMID: 16140971
-
Role of epithelial nitric oxide in airway viral infection.Free Radic Biol Med. 2006 Jul 1;41(1):19-28. doi: 10.1016/j.freeradbiomed.2006.01.037. Epub 2006 Feb 20. Free Radic Biol Med. 2006. PMID: 16781449 Free PMC article. Review.
Cited by
-
Comparative transcriptome analysis unveils the adaptative mechanisms of Scedosporium apiospermum to the microenvironment encountered in the lungs of patients with cystic fibrosis.Comput Struct Biotechnol J. 2020 Nov 10;18:3468-3483. doi: 10.1016/j.csbj.2020.10.034. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33294141 Free PMC article.
-
Regulatory role of β-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells.J Lipid Res. 2012 Jul;53(7):1268-76. doi: 10.1194/jlr.M021972. Epub 2012 Apr 22. J Lipid Res. 2012. PMID: 22523395 Free PMC article.
-
Regulation of airway tight junctions by proinflammatory cytokines.Mol Biol Cell. 2002 Sep;13(9):3218-34. doi: 10.1091/mbc.e02-03-0134. Mol Biol Cell. 2002. PMID: 12221127 Free PMC article.
-
Genomewide association analysis of respiratory syncytial virus infection in mice.J Virol. 2010 Mar;84(5):2257-69. doi: 10.1128/JVI.00584-09. Epub 2009 Dec 16. J Virol. 2010. PMID: 20015999 Free PMC article.
-
Pulmonary inflammation induced by Pseudomonas aeruginosa lipopolysaccharide, phospholipase C, and exotoxin A: role of interferon regulatory factor 1.Infect Immun. 2002 Mar;70(3):1352-8. doi: 10.1128/IAI.70.3.1352-1358.2002. Infect Immun. 2002. PMID: 11854220 Free PMC article.
References
-
- Welsh, M.J., Tsui, L.C., Boat, T.F., and Beaudet, A.L. 1995. Cystic fibrosis. In The metabolic and molecular basis of inherited disease. 7th edition. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, New York. 3799–3863.
-
- Meng Q-H, et al. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol. 1998;184:323–331. - PubMed
-
- Elmer HL, Brady KG, Drumm ML, Kelley TJ. Nitric oxide-mediated regulation of transepithelial sodium and chloride transport in murine nasal epithelium. Am J Physiol. 1999;276:L466–L473. - PubMed
-
- Kroesbergen A, Jobsis Q, Bel EH, Hop WC, de Jongste JC. Flow-dependency of exhaled nitric oxide in children with asthma and cystic fibrosis. Eur Respir J. 1999;14:871–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

