Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice
- PMID: 10930444
- PMCID: PMC314325
- DOI: 10.1172/JCI9225
Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice
Erratum in
- J Clin Invest 2000 Sep;106(5):715
Abstract
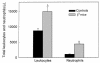
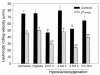
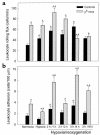
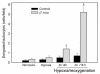
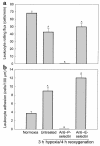
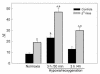
In sickle cell anemia, the initiation, progression, and resolution of a vasoocclusive episode may present features of ischemia-reperfusion injury, with recurrent episodes of ischemia/hypoxia and reoxygenation promoting inflammation. Here, we have tested the hypothesis that hypoxia/reoxygenation triggers inflammation in the transgenic sickle mouse. In these mice, even at ambient air, peripheral leukocyte counts are elevated by 1.7-fold and neutrophil counts by almost 3-fold. Two hours of hypoxia, followed by reoxygenation, induced a greater than normal rolling flux and adhesion of leukocytes in these mice, but no leukocyte extravasation. When 3 hours of hypoxia was followed by reoxygenation, sickle mice, but not normal mice, showed a distinct inflammatory response characterized by an increased number of adherent and emigrated leukocytes. Because these events, which are exaggerated in sickle mice, are not seen in response to hypoxia alone, we conclude that they represent a form of reperfusion injury. Studies using an H(2)O(2)-sensitive probe revealed clear evidence of oxidant production in vascular endothelial cells after hypoxia/reoxygenation in sickle mice. Infusion of an anti-P-selectin antibody, but not an anti-E-selectin antibody, completely inhibited this inflammatory response and significantly increased wall shear rates. These findings suggest that leukocyte-endothelium interaction contribute to vasoocclusive events in the sickle mice and perhaps in human sickle disease.
Figures

Comment in
-
Sickle cell anemia as an inflammatory disease.J Clin Invest. 2000 Aug;106(3):337-8. doi: 10.1172/JCI10726. J Clin Invest. 2000. PMID: 10930436 Free PMC article. No abstract available.
References
-
- Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. - PubMed
-
- Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev. 1996;10:29–44. - PubMed
-
- Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med. 1998;25:404–433. - PubMed
-
- Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. - PubMed
-
- Klug PP, Kaye N, Jensen WN. Endothelial cell and vascular damage in the sickle cell disorders. Blood Cells. 1982;8:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

