Analysis of cortical flow models in vivo
- PMID: 10930453
- PMCID: PMC14939
- DOI: 10.1091/mbc.11.8.2553
Analysis of cortical flow models in vivo
Abstract
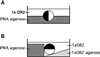
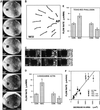
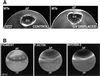
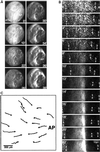
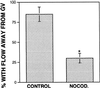
Cortical flow, the directed movement of cortical F-actin and cortical organelles, is a basic cellular motility process. Microtubules are thought to somehow direct cortical flow, but whether they do so by stimulating or inhibiting contraction of the cortical actin cytoskeleton is the subject of debate. Treatment of Xenopus oocytes with phorbol 12-myristate 13-acetate (PMA) triggers cortical flow toward the animal pole of the oocyte; this flow is suppressed by microtubules. To determine how this suppression occurs and whether it can control the direction of cortical flow, oocytes were subjected to localized manipulation of either the contractile stimulus (PMA) or microtubules. Localized PMA application resulted in redirection of cortical flow toward the site of application, as judged by movement of cortical pigment granules, cortical F-actin, and cortical myosin-2A. Such redirected flow was accelerated by microtubule depolymerization, showing that the suppression of cortical flow by microtubules is independent of the direction of flow. Direct observation of cortical F-actin by time-lapse confocal analysis in combination with photobleaching showed that cortical flow is driven by contraction of the cortical F-actin network and that microtubules suppress this contraction. The oocyte germinal vesicle serves as a microtubule organizing center in Xenopus oocytes; experimental displacement of the germinal vesicle toward the animal pole resulted in localized flow away from the animal pole. The results show that 1) cortical flow is directed toward areas of localized contraction of the cortical F-actin cytoskeleton; 2) microtubules suppress cortical flow by inhibiting contraction of the cortical F-actin cytoskeleton; and 3) localized, microtubule-dependent suppression of actomyosin-based contraction can control the direction of cortical flow. We discuss these findings in light of current models of cortical flow.
Figures


References
-
- Bement WM. Signal transduction by calcium and protein kinase C during egg activation. J Exp Zool. 1992;263:382–397. - PubMed
-
- Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. - PubMed
-
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. - PubMed
-
- Bluemink JG. Cortical wound healing in the amphibian egg. J Ultrastruct Res. 1972;41:95–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

