Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89
- PMID: 10930455
- PMCID: PMC14941
- DOI: 10.1091/mbc.11.8.2577
Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89
Abstract
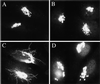
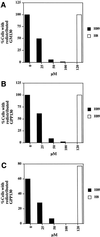
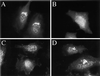
Recent evidence suggests a regulatory connection between cell volume, endoplasmic reticulum (ER) export, and stimulated Golgi-to-ER transport. To investigate the potential role of protein kinases we tested a panel of protein kinase inhibitors for their effect on these steps. One inhibitor, H89, an isoquinolinesulfonamide that is commonly used as a selective protein kinase A inhibitor, blocked both ER export and hypo-osmotic-, brefeldin A-, or nocodazole-induced Golgi-to-ER transport. In contrast, H89 did not block the constitutive ER Golgi-intermediate compartment (ERGIC)-to-ER and Golgi-to-ER traffic that underlies redistribution of ERGIC and Golgi proteins into the ER after ER export arrest. Surprisingly, other protein kinase A inhibitors, KT5720 and H8, as well as a set of protein kinase C inhibitors, had no effect on these transport processes. To test whether H89 might act at the level of either the coatomer protein (COP)I or the COPII coat protein complex we examined the localization of betaCOP and Sec13 in H89-treated cells. H89 treatment led to a rapid loss of Sec13-labeled ER export sites but betaCOP localization to the Golgi was unaffected. To further investigate the effect of H89 on COPII we developed a COPII recruitment assay with permeabilized cells and found that H89 potently inhibited binding of exogenous Sec13 to ER export sites. This block occurred in the presence of guanosine-5'-O-(3-thio)triphosphate, suggesting that Sec13 recruitment is inhibited at a step independent of the activation of the GTPase Sar1. These results identify a requirement for an H89-sensitive factor(s), potentially a novel protein kinase, in recruitment of COPII to ER export sites, as well as in stimulated but not constitutive Golgi-to-ER transport.
Figures





Similar articles
-
Sar1 translocation onto the ER-membrane for vesicle budding has different pathways for promotion and suppression of ER-to-Golgi transport mediated through H89-sensitive kinase and ER-resident G protein.Mol Cell Biochem. 2012 Jul;366(1-2):175-82. doi: 10.1007/s11010-012-1295-x. Epub 2012 Apr 7. Mol Cell Biochem. 2012. PMID: 22484643
-
Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum.J Biol Chem. 2000 Nov 17;275(46):35673-6. doi: 10.1074/jbc.C000449200. J Biol Chem. 2000. PMID: 11001944
-
Maintenance of Golgi structure and function depends on the integrity of ER export.J Cell Biol. 2001 Nov 12;155(4):557-70. doi: 10.1083/jcb.200107045. Epub 2001 Nov 12. J Cell Biol. 2001. PMID: 11706049 Free PMC article.
-
Protein and lipid sorting between the endoplasmic reticulum and the Golgi complex.Semin Cell Dev Biol. 1998 Oct;9(5):493-501. doi: 10.1006/scdb.1998.0256. Semin Cell Dev Biol. 1998. PMID: 9835636 Review.
-
A structural view of the COPII vesicle coat.Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review.
Cited by
-
The cytoplasmic accumulations of the cataract-associated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum.Exp Eye Res. 2009 Mar;88(3):600-9. doi: 10.1016/j.exer.2008.11.024. Epub 2008 Dec 6. Exp Eye Res. 2009. PMID: 19073179 Free PMC article.
-
A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo.Mol Cancer Ther. 2010 May;9(5):1136-46. doi: 10.1158/1535-7163.MCT-09-1145. Epub 2010 May 4. Mol Cancer Ther. 2010. PMID: 20442301 Free PMC article.
-
COPII machinery cooperates with ER-localized Hsp40 to sequester misfolded membrane proteins into ER-associated compartments.Mol Biol Cell. 2013 Mar;24(5):633-42. doi: 10.1091/mbc.E12-08-0639. Epub 2013 Jan 9. Mol Biol Cell. 2013. PMID: 23303252 Free PMC article.
-
Mouse hepatitis coronavirus RNA replication depends on GBF1-mediated ARF1 activation.PLoS Pathog. 2008 Jun 13;4(6):e1000088. doi: 10.1371/journal.ppat.1000088. PLoS Pathog. 2008. PMID: 18551169 Free PMC article.
-
PITPβ promotes COPI vesicle fission through lipid transfer and membrane contact formation.J Cell Biol. 2025 May 5;224(5):e202407166. doi: 10.1083/jcb.202407166. Epub 2025 Apr 11. J Cell Biol. 2025. PMID: 40214667
References
-
- Barlowe C, d'Enfert C, Schekman R. Purification and characterization of Sar1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;15:873–879. - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M, Razzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by SEC proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Bednarak SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI-coated and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. - PubMed
-
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

