The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis
- PMID: 10930471
- PMCID: PMC14957
- DOI: 10.1091/mbc.11.8.2803
The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis
Abstract
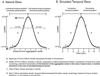
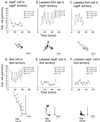
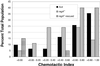
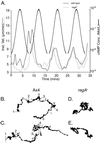
Dictyostelium strains in which the gene encoding the cytoplasmic cAMP phosphodiesterase RegA is inactivated form small aggregates. This defect was corrected by introducing copies of the wild-type regA gene, indicating that the defect was solely the consequence of the loss of the phosphodiesterase. Using a computer-assisted motion analysis system, regA(-) mutant cells were found to show little sense of direction during aggregation. When labeled wild-type cells were followed in a field of aggregating regA(-) cells, they also failed to move in an orderly direction, indicating that signaling was impaired in mutant cell cultures. However, when labeled regA(-) cells were followed in a field of aggregating wild-type cells, they again failed to move in an orderly manner, primarily in the deduced fronts of waves, indicating that the chemotactic response was also impaired. Since wild-type cells must assess both the increasing spatial gradient and the increasing temporal gradient of cAMP in the front of a natural wave, the behavior of regA(-) cells was motion analyzed first in simulated temporal waves in the absence of spatial gradients and then was analyzed in spatial gradients in the absence of temporal waves. Our results demonstrate that RegA is involved neither in assessing the direction of a spatial gradient of cAMP nor in distinguishing between increasing and decreasing temporal gradients of cAMP. However, RegA is essential for specifically suppressing lateral pseudopod formation during the response to an increasing temporal gradient of cAMP, a necessary component of natural chemotaxis. We discuss the possibility that RegA functions in a network that regulates myosin phosphorylation by controlling internal cAMP levels, and, in support of that hypothesis, we demonstrate that myosin II does not localize in a normal manner to the cortex of regA(-) cells in an increasing temporal gradient of cAMP.
Figures






Similar articles
-
Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis.Eukaryot Cell. 2005 Apr;4(4):775-86. doi: 10.1128/EC.4.4.775-786.2005. Eukaryot Cell. 2005. PMID: 15821137 Free PMC article.
-
Constitutively active protein kinase A disrupts motility and chemotaxis in Dictyostelium discoideum.Eukaryot Cell. 2003 Feb;2(1):62-75. doi: 10.1128/EC.2.1.62-75.2003. Eukaryot Cell. 2003. PMID: 12582123 Free PMC article.
-
RasC plays a role in transduction of temporal gradient information in the cyclic-AMP wave of Dictyostelium discoideum.Eukaryot Cell. 2004 Jun;3(3):646-62. doi: 10.1128/EC.3.3.646-662.2004. Eukaryot Cell. 2004. PMID: 15189986 Free PMC article.
-
A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum.J Muscle Res Cell Motil. 2002;23(7-8):659-72. doi: 10.1023/a:1024459124427. J Muscle Res Cell Motil. 2002. PMID: 12952065 Review.
-
Streamer F mutants and chemotaxis of Dictyostelium.Bioessays. 1992 Jul;14(7):473-9. doi: 10.1002/bies.950140708. Bioessays. 1992. PMID: 1332700 Review.
Cited by
-
Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis.Eukaryot Cell. 2005 Apr;4(4):775-86. doi: 10.1128/EC.4.4.775-786.2005. Eukaryot Cell. 2005. PMID: 15821137 Free PMC article.
-
Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium.Mol Biol Cell. 2005 Sep;16(9):4256-66. doi: 10.1091/mbc.e05-03-0219. Epub 2005 Jun 29. Mol Biol Cell. 2005. PMID: 15987738 Free PMC article.
-
cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection.J Muscle Res Cell Motil. 2002;23(7-8):793-802. doi: 10.1023/a:1024483829878. J Muscle Res Cell Motil. 2002. PMID: 12952077 Review.
-
Taxis equations for amoeboid cells.J Math Biol. 2007 Jun;54(6):847-85. doi: 10.1007/s00285-007-0070-1. Epub 2007 Feb 2. J Math Biol. 2007. PMID: 17273880
-
The N-terminus of Dictyostelium Scar interacts with Abi and HSPC300 and is essential for proper regulation and function.Mol Biol Cell. 2007 May;18(5):1609-20. doi: 10.1091/mbc.e06-06-0518. Epub 2007 Feb 21. Mol Biol Cell. 2007. PMID: 17314411 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

