Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome
- PMID: 10930472
- PMCID: PMC14958
- DOI: 10.1091/mbc.11.8.2821
Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome
Abstract
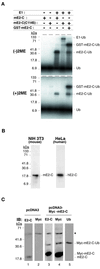
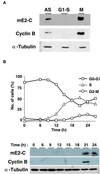
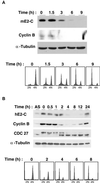
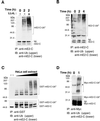
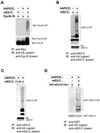
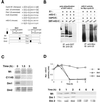
Progression through mitosis requires the precisely timed ubiquitin-dependent degradation of specific substrates. E2-C is a ubiquitin-conjugating enzyme that plays a critical role with anaphase-promoting complex/cyclosome (APC/C) in progression of and exit from M phase. Here we report that mammalian E2-C is expressed in late G(2)/M phase and is degraded as cells exit from M phase. The mammalian E2-C shows an autoubiquitinating activity leading to covalent conjugation to itself with several ubiquitins. The ubiquitination of E2-C is strongly enhanced by APC/C, resulting in the formation of a polyubiquitin chain. The polyubiquitination of mammalian E2-C occurs only when cells exit from M phase. Furthermore, mammalian E2-C contains two putative destruction boxes that are believed to act as recognition motifs for APC/C. The mutation of this motif reduced the polyubiquitination of mammalian E2-C, resulting in its stabilization. These results suggest that mammalian E2-C is itself a substrate of the APC/C-dependent proteolysis machinery, and that the periodic expression of mammalian E2-C may be a novel autoregulatory system for the control of the APC/C activity and its substrate specificity.
Figures
Similar articles
-
The APC11 RING-H2 finger mediates E2-dependent ubiquitination.Mol Biol Cell. 2000 Jul;11(7):2315-25. doi: 10.1091/mbc.11.7.2315. Mol Biol Cell. 2000. PMID: 10888670 Free PMC article.
-
Subunits and substrates of the anaphase-promoting complex.Exp Cell Res. 1999 May 1;248(2):339-49. doi: 10.1006/excr.1999.4443. Exp Cell Res. 1999. PMID: 10222126 Review.
-
APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex.Mol Biol Cell. 2001 Dec;12(12):3839-51. doi: 10.1091/mbc.12.12.3839. Mol Biol Cell. 2001. PMID: 11739784 Free PMC article.
-
A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression.Mol Cell Biol. 2001 Jun;21(11):3692-703. doi: 10.1128/MCB.21.11.3692-3703.2001. Mol Cell Biol. 2001. PMID: 11340163 Free PMC article.
-
Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization.Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1559-69; discussion 1569-70. doi: 10.1098/rstb.1999.0499. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582241 Free PMC article. Review.
Cited by
-
Ubiquitin-specific protease 7 is a regulator of ubiquitin-conjugating enzyme UbE2E1.J Biol Chem. 2013 Jun 7;288(23):16975-16985. doi: 10.1074/jbc.M113.469262. Epub 2013 Apr 19. J Biol Chem. 2013. PMID: 23603909 Free PMC article.
-
UbcH10 a Major Actor in Cancerogenesis and a Potential Tool for Diagnosis and Therapy.Int J Mol Sci. 2020 Mar 17;21(6):2041. doi: 10.3390/ijms21062041. Int J Mol Sci. 2020. PMID: 32192022 Free PMC article. Review.
-
Expression and effect of inhibition of the ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma.Neoplasia. 2006 Dec;8(12):1062-71. doi: 10.1593/neo.05832. Neoplasia. 2006. PMID: 17217624 Free PMC article.
-
Time-lapse imaging of neuroblastoma cells to determine cell fate upon gene knockdown.PLoS One. 2012;7(12):e50988. doi: 10.1371/journal.pone.0050988. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251412 Free PMC article.
-
The PAR2 signal peptide prevents premature receptor cleavage and activation.PLoS One. 2020 Feb 20;15(2):e0222685. doi: 10.1371/journal.pone.0222685. eCollection 2020. PLoS One. 2020. Retraction in: PLoS One. 2024 Jun 6;19(6):e0305363. doi: 10.1371/journal.pone.0305363. PMID: 32078628 Free PMC article. Retracted.
References
-
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Arvand A, Bastians H, Welford SM, Thompson AD, Ruderman JV, Denny CT. EWS/FLI1 up regulates mE2-C, a cyclin-selective ubiquitin conjugating enzyme involved in cyclin B destruction. Oncogene. 1998;17:2039–2045. - PubMed
-
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
-
- Banerjee A, Gregori L, Xu Y, Chau V. The bacterially expressed yeast CDC34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. J Biol Chem. 1993;268:5668–5675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

