Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir
- PMID: 10930961
- PMCID: PMC2014393
- DOI: 10.1046/j.1365-2125.2000.00245.x
Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir
Abstract
Aims: To investigate the effect of the antiretroviral protease inhibitors saquinavir (soft gelatin capsule) and ritonavir on the pharmacokinetic properties and tolerability of sildenafil and to investigate the effect of sildenafil on the steady-state pharmacokinetics of saquinavir and ritonavir.
Methods: Two independent, 8 day, open, randomized, placebo-controlled, parallel-group studies (containing a double-blind crossover phase) were conducted at Pfizer Clinical research units (Canterbury, UK. and Brussels, Belgium). Twenty-eight healthy male volunteers entered each study. In each study, volunteers were randomized (n = 14 per group) to receive sildenafil on day 1 followed by a 7-day treatment period (days 2-8) with saquinavir or placebo (Study I) or ritonavir or placebo (Study II). Sildenafil or placebo (Study I and Study II) was administered alternately on day 7 or day 8, depending on initial randomization. The effect of saquinavir and ritonavir on the pharmacokinetics of sildenafil and its primary circulating metabolite (UK-103, 320) and the effect of single-dose sildenafil on the steady-state pharmacokinetics of saquinavir (1200 mg three times daily) and ritonavir (500 mg twice daily) were determined. The safety and tolerability of sildenafil coadministered with saquinavir or ritonavir were also assessed.
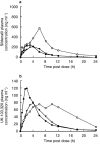
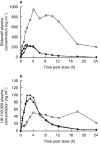
Results: Both protease inhibitors significantly increased Cmax, AUC, tmax and t(1/2) values for both sildenafil and UK-103, 320. Ritonavir showed a significantly greater effect than saquinavir with increases in sildenafil AUC and Cmax of 11-fold (95% CI: 9.0, 12.0) and 3.9-fold (95% CI: 3.2, 4.9), respectively. This compared with increases of 3.1-fold (95% CI: 2.5, 4.0) and 2.4-fold (95% CI: 1.8, 3.3) for coadministration with saquinavir. In contrast, the steady-state pharmacokinetics of saquinavir and ritonavir were unaffected by sildenafil. The increases in systemic exposure to sildenafil and UK-103, 320 were not associated with an increased incidence of adverse events or clinically significant changes in blood pressure, heart rate or ECG parameters.
Conclusions: These results indicate that both saquinavir and ritonavir modify the pharmacokinetics of sildenafil presumably through inhibition of CYP3A4. The more pronounced effect of ritonavir may be attributed to its additional potent inhibition of CYP2C9. No change in safety or tolerability was observed when sildenafil was coadministered with either protease inhibitor. However, given the extent of the interactions, a lower sildenafil starting dose (25 mg) should be considered for patients receiving saquinavir and it is recommended not to exceed a maximum single dose of 25 mg in a 48 h period for patients receiving ritonavir.
Figures
Comment in
-
Re: 'Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir'.Br J Clin Pharmacol. 2000 Aug;50(2):85. doi: 10.1046/j.1365-2125.2000.00246.x. Br J Clin Pharmacol. 2000. PMID: 10930959 Free PMC article. No abstract available.
Similar articles
-
Effect of repeated doses of darunavir plus low-dose ritonavir on the pharmacokinetics of sildenafil in healthy male subjects: phase I randomized, open-label, two-way crossover study.Clin Drug Investig. 2008;28(8):479-85. doi: 10.2165/00044011-200828080-00002. Clin Drug Investig. 2008. PMID: 18598093 Clinical Trial.
-
The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers.Br J Clin Pharmacol. 2002;53 Suppl 1(Suppl 1):37S-43S. doi: 10.1046/j.0306-5251.2001.00031.x. Br J Clin Pharmacol. 2002. PMID: 11879258 Free PMC article. Clinical Trial.
-
Interaction of sildenafil and indinavir when co-administered to HIV-positive patients.AIDS. 1999 Oct 22;13(15):F101-7. doi: 10.1097/00002030-199910220-00001. AIDS. 1999. PMID: 10546851
-
Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents.Clin Pharmacokinet. 1998 Oct;35(4):275-91. doi: 10.2165/00003088-199835040-00002. Clin Pharmacokinet. 1998. PMID: 9812178 Review.
-
Saquinavir: a review of its use in boosted regimens for treating HIV infection.Drugs. 2003;63(12):1299-324. doi: 10.2165/00003495-200363120-00007. Drugs. 2003. PMID: 12790697 Review.
Cited by
-
Urological aspects of HIV and AIDS.Nat Rev Urol. 2013 Dec;10(12):713-22. doi: 10.1038/nrurol.2013.230. Epub 2013 Oct 29. Nat Rev Urol. 2013. PMID: 24166342 Review.
-
Sildenafil and bosentan plasma concentrations in a human immunodeficiency virus- infected patient with pulmonary arterial hypertension treated with ritonavir-boosted protease inhibitor.Infect Dis Rep. 2015 Mar 16;7(1):5822. doi: 10.4081/idr.2015.5822. eCollection 2015 Feb 24. Infect Dis Rep. 2015. PMID: 25874072 Free PMC article.
-
Pulmonary Arterial Hypertension among HIV-Infected Children: Results of a National Survey and Review of the Literature.Front Pediatr. 2015 Apr 7;3:25. doi: 10.3389/fped.2015.00025. eCollection 2015. Front Pediatr. 2015. PMID: 25905096 Free PMC article. Review.
-
Reduction of saquinavir exposure by coadministration of loperamide: a two-way pharmacokinetic interaction.Clin Pharmacokinet. 2004;43(14):1015-24. doi: 10.2165/00003088-200443140-00004. Clin Pharmacokinet. 2004. PMID: 15530130 Clinical Trial.
-
Hardness, function, emotional well-being, satisfaction and the overall sexual experience in men using 100-mg fixed-dose or flexible-dose sildenafil citrate.Int J Impot Res. 2010 Jul-Aug;22(4):284-9. doi: 10.1038/ijir.2010.17. Epub 2010 Jul 1. Int J Impot Res. 2010. PMID: 20596083 Free PMC article.
References
-
- Goldstein I, Lau TF, Padma‐nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. - PubMed
-
- Langtry HD, Markham A, Sildenafil A review of its use in erectile dysfunction. Drugs. 1999;57:967–989. - PubMed
-
- Goldenberg MM. Safety and efficacy of sildenafil citrate in the treatment of male erectile dysfunction. Clin Ther. 1998;20:1033–1048. - PubMed
-
- US. Prescribing Information. New York: Pfizer Inc.; 1998.
-
- Catalan J, Klimes I, Bond A, Day A, Garrod A, Rizza C. The psychosocial impact of HIV infection in men with haemophilia: controlled investigation and factors associated with psychiatric morbidity. J Psychosom Res. 1992;36:409–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

