Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis
- PMID: 10930972
- PMCID: PMC2014399
- DOI: 10.1111/j.1365-2125.2000.00179.x
Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis
Abstract
Aims: Experimental studies have suggested that constant intravenous infusion would be preferable to conventional intermittent bolus administration of beta-lactam antibiotics for serious Gram-negative infections. Severe melioidosis (Burkholderia pseudomallei infection) carries a mortality over 40% despite treatment with high dose ceftazidime. The aim of this study was to measure the pharmacokinetic and pharmacodynamic effects of continuous infusion of ceftazidime vs intermittent bolus dosing in septicaemic melioidosis.
Methods: Patients with suspected septicaemic melioidosis were randomised to receive ceftazidime 40 mg kg(-1) 8 hourly by bolus injection or 4 mg kg(-1) h(-1) by constant infusion following a 12 mg kg(-1) priming dose and pharmacokinetic and pharmacodynamic parameters were compared.
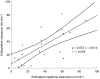
Results: Of the 34 patients studied 16 (59%) died. Twenty patients had cultures positive for B. pseudomallei of whom 12 (60%) died. The median MIC90 of B. pseudomallei was 2 mg l(-1), giving a minimum target concentration (4*MIC) of 8 mg l(-1). The median (range) estimated total apparent volume of distribution, systemic clearance and terminal elimination half-lives of ceftazidime were 0.468 (0.241-0. 573) l kg(-1), 0.058 (0.005-0.159) l kg(-1) h(-1) and 7.74 (1.95-44.71) h, respectively. Clearance of ceftazidime and creatinine clearance were correlated closely (r = 0.71; P < 0.001) and there was no evidence of significant nonrenal clearance.
Conclusions: Simulations based on these data and the ceftazidime sensitivity of the B. pseudomallei isolates indicated that administration by constant infusion would allow significant dose reduction and cost saving. With conventional 8 h intermittent dosing to patients with normal renal function, plasma ceftazidime concentrations could fall below the target concentration but this would be unlikely with a constant infusion. Correction for renal failure, which is common in patients with meliodosis is Clearance = k(*) creatinine clearance where k = 0.72. Calculation of a loading dose gives median (range) values of loading dose, DL of 18.7 mg kg(-1) (9.5-23) and infusion rate I = 3.5 mg k(-1) h(-1) (0.4-13) (which equals 84 mg kg(-1) day(-1)). A nomogram for adjustment in renal failure is given.
Figures

Corrected and republished from
-
Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis.Br J Clin Pharmacol. 2000 May;49(5):445-52. doi: 10.1046/j.1365-2125.2000.00179.x. Br J Clin Pharmacol. 2000. Corrected and republished in: Br J Clin Pharmacol. 2000 Aug;50(2):184-91. doi: 10.1111/j.1365-2125.2000.00179.x. PMID: 10792202 Free PMC article. Corrected and republished. Clinical Trial.
References
-
- Mattie H, Craig WA, Pechere JC. Determinants of efficacy and toxicity of aminoglycosides. J Antimicrob Chemother. 1989;24:281–293. - PubMed
-
- Vogelman B, Gudmundsson S, Leggett J. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. - PubMed
-
- Miglioli P, Xerri L, Palatini P. Influence of the mode of administration on the penetration of ceftazidime into tissues and pleural exudate of rats. Pharmacol. 1991;43:242–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

