Isolation and characterization of cDNA encoding the antigenic protein of the human tRNP(Ser)Sec complex recognized by autoantibodies from patients withtype-1 autoimmune hepatitis
- PMID: 10931155
- PMCID: PMC1905695
- DOI: 10.1046/j.1365-2249.2000.01280.x
Isolation and characterization of cDNA encoding the antigenic protein of the human tRNP(Ser)Sec complex recognized by autoantibodies from patients withtype-1 autoimmune hepatitis
Abstract
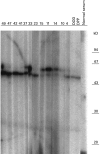
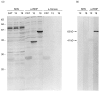
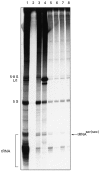
We previously described autoantibodies against a UGA serine tRNA-protein complex (tRNP(Ser)Sec) in patients with type-1 autoimmune hepatitis [1] and now define the specificity and frequency of this autoantibody and the DNA sequence encoding the tRNA(Ser)Sec-associated antigenic protein. The presence of anti-tRNP(Ser)Sec antibodies was highly specific for type-1 autoimmune hepatitis, as 47.5% of patients were positive compared with none of the control subjects. To characterize the antigenic protein(s), we immunoscreened a human cDNA library with anti-tRNP(Ser)Sec-positive sera. Two clones (19 and 13) were isolated. Clone 19 encodes a protein with a predicted molecular mass of 48.8 kD. Clone 13 is a shorter cDNA, almost identical to clone 19, which encodes a 35.9-kD protein. Expression of both cDNAs was accomplished in Escherichia coli as His-tagged recombinant proteins. Antibodies eluted from both purified recombinant proteins were able to immunoprecipitate the tRNA(Ser)Sec from a HeLa S3 cell extract, demonstrating their cross-reactivity with the mammalian antigenic complex. Recent cloning data relating to the target antigen(s) of autoantibodies in autoimmune hepatitis patients that react with a soluble liver antigen (SLA) and a liver-pancreas antigen (LP) have revealed that these two autoantibodies are identical and that the cloned antigen shows 99% amino acid sequence homology with tRNP(Ser)Sec.
Figures


References
-
- Maddrey WC. Subdivisions of idiopathic autoimmune chronic active hepatitis. Hepatology. 1987;7:1372–5. - PubMed
-
- Maddrey WC, Willis C. Chronic hepatitis. In: Bone RC, editor. Disease-a-month. XXXIX. 1993. pp. 53–126. No. 2. - PubMed
-
- Manns M. Autoantibodies and antigens in liver diseases-updated. J Hepatol. 1989;9:272–80. - PubMed
-
- Czaja AJ. Autoantibodies. Bailliere's Clin Gastroenterol. 1995;9:723–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

