Tracking COL1A1 RNA in osteogenesis imperfecta. splice-defective transcripts initiate transport from the gene but are retained within the SC35 domain
- PMID: 10931857
- PMCID: PMC2175183
- DOI: 10.1083/jcb.150.3.417
Tracking COL1A1 RNA in osteogenesis imperfecta. splice-defective transcripts initiate transport from the gene but are retained within the SC35 domain
Abstract
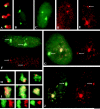
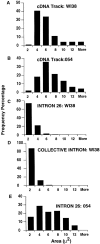
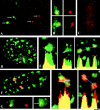
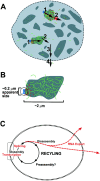
This study illuminates the intra-nuclear fate of COL1A1 RNA in osteogenesis imperfecta (OI) Type I. Patient fibroblasts were shown to carry a heterozygous defect in splicing of intron 26, blocking mRNA export. Both the normal and mutant allele associated with a nuclear RNA track, a localized accumulation of posttranscriptional RNA emanating to one side of the gene. Both tracks had slightly elongated or globular morphology, but mutant tracks were cytologically distinct in that they lacked the normal polar distribution of intron 26. Normal COL1A1 RNA tracks distribute throughout an SC-35 domain, from the gene at the periphery. Normally, almost all 50 COL1A1 introns are spliced at or adjacent to the gene, before mRNA transits thru the domain. Normal COL1A1 transcripts may undergo maturation needed for export within the domain such as removal of a slow-splicing intron (shown for intron 24), after which they may disperse. Splice-defective transcripts still distribute thru the SC-35 domain, moving approximately 1-3 micrometer from the gene. However, microfluorimetric analyses demonstrate mutant transcripts accumulate to abnormal levels within the track and domain. Hence, mutant transcripts initiate transport from the gene, but are impeded in exit from the SC-35 domain. This identifies a previously undefined step in mRNA export, involving movement through an SC-35 domain. A model is presented in which maturation and release for export of COL1A1 mRNA is linked to rapid cycling of metabolic complexes within the splicing factor domain, adjacent to the gene. This paradigm may apply to SC-35 domains more generally, which we suggest may be nucleated at sites of high demand and comprise factors being actively used to facilitate expression of associated loci.
Figures


Similar articles
-
Nuclear pre-mRNA compartmentalization: trafficking of released transcripts to splicing factor reservoirs.Mol Biol Cell. 2000 Feb;11(2):497-510. doi: 10.1091/mbc.11.2.497. Mol Biol Cell. 2000. PMID: 10679009 Free PMC article.
-
Redefinition of exon 7 in the COL1A1 gene of type I collagen by an intron 8 splice-donor-site mutation in a form of osteogenesis imperfecta: influence of intron splice order on outcome of splice-site mutation.Am J Hum Genet. 1999 Aug;65(2):336-44. doi: 10.1086/302512. Am J Hum Genet. 1999. PMID: 10417276 Free PMC article.
-
Defective splicing of mRNA from one COL1A1 allele of type I collagen in nondeforming (type I) osteogenesis imperfecta.J Clin Invest. 1993 Oct;92(4):1994-2002. doi: 10.1172/JCI116794. J Clin Invest. 1993. PMID: 8408653 Free PMC article.
-
Nuclear mechanisms of gene expression control: pre-mRNA splicing as a life or death decision.Curr Opin Genet Dev. 2021 Apr;67:67-76. doi: 10.1016/j.gde.2020.11.002. Epub 2020 Dec 5. Curr Opin Genet Dev. 2021. PMID: 33291060 Free PMC article. Review.
-
Nuclear organization and gene expression.Exp Cell Res. 1996 Dec 15;229(2):189-97. doi: 10.1006/excr.1996.0358. Exp Cell Res. 1996. PMID: 8986596 Review.
Cited by
-
AURKB-mediated effects on chromatin regulate binding versus release of XIST RNA to the inactive chromosome.J Cell Biol. 2009 Aug 24;186(4):491-507. doi: 10.1083/jcb.200811143. J Cell Biol. 2009. PMID: 19704020 Free PMC article.
-
Pathological phase transitions in ALS-FTD impair dynamic RNA-protein granules.RNA. 2022 Jan;28(1):97-113. doi: 10.1261/rna.079001.121. Epub 2021 Oct 27. RNA. 2022. PMID: 34706979 Free PMC article. Review.
-
Large-scale chromatin structure of inducible genes: transcription on a condensed, linear template.J Cell Biol. 2009 Apr 6;185(1):87-100. doi: 10.1083/jcb.200809196. J Cell Biol. 2009. PMID: 19349581 Free PMC article.
-
RNA as a fundamental component of interphase chromosomes: could repeats prove key?Curr Opin Genet Dev. 2016 Apr;37:137-147. doi: 10.1016/j.gde.2016.04.005. Epub 2016 May 21. Curr Opin Genet Dev. 2016. PMID: 27218204 Free PMC article. Review.
-
In vivo BiFC analysis of Y14 and NXF1 mRNA export complexes: preferential localization within and around SC35 domains.J Cell Biol. 2006 Jan 30;172(3):373-81. doi: 10.1083/jcb.200503061. Epub 2006 Jan 23. J Cell Biol. 2006. PMID: 16431928 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

