The dynamics of postmitotic reassembly of the nucleolus
- PMID: 10931858
- PMCID: PMC2175201
- DOI: 10.1083/jcb.150.3.433
The dynamics of postmitotic reassembly of the nucleolus
Abstract
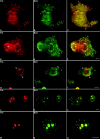
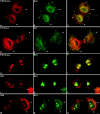
Mammalian cell nucleoli disassemble at the onset of M-phase and reassemble during telophase. Recent studies showed that partially processed preribosomal RNA (pre-rRNA) is preserved in association with processing components in the perichromosomal regions (PRs) and in particles called nucleolus-derived foci (NDF) during mitosis. Here, the dynamics of nucleolar reassembly were examined for the first time in living cells expressing fusions of the processing-related proteins fibrillarin, nucleolin, or B23 with green fluorescent protein (GFP). During telophase the NDF disappeared with a concomitant appearance of material in the reforming nuclei. Prenucleolar bodies (PNBs) appeared in nuclei in early telophase and gradually disappeared as nucleoli formed, strongly suggesting the transfer of PNB components to newly forming nucleoli. Fluorescence recovery after photobleaching (FRAP) showed that fibrillarin-GFP reassociates with the NDF and PNBs at rapid and similar rates. The reentry of processing complexes into telophase nuclei is suggested by the presence of pre-rRNA sequences in PNBs. Entry of specific proteins into the nucleolus approximately correlated with the timing of processing events. The mitotically preserved processing complexes may be essential for regulating the distribution of components to reassembling daughter cell nucleoli.
Figures




References
-
- Azum-Gélade M.C., Noaillac-Depeyre J., Caizergues-Ferrer M., Gas N. Cell cycle redistribution of U3 snRNA and fibrillarin. Presence in the cytoplasmic nucleolus remnant and in the prenucleolar bodies at telophase. J. Cell Sci. 1994;107:463–475. - PubMed
-
- Bell P., Scheer U. Prenucleolar bodies contain coilin and are assembled in Xenopus egg extract depleted of specific nucleolar proteins and U3 RNA. J. Cell Sci. 1996;109:43–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

