Visualization of tRNA movements on the Escherichia coli 70S ribosome during the elongation cycle
- PMID: 10931859
- PMCID: PMC2175196
- DOI: 10.1083/jcb.150.3.447
Visualization of tRNA movements on the Escherichia coli 70S ribosome during the elongation cycle
Abstract
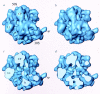
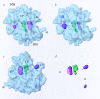
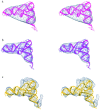
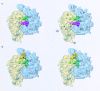
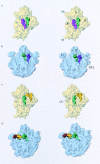
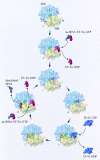
Three-dimensional cryomaps have been reconstructed for tRNA-ribosome complexes in pre- and posttranslocational states at 17-A resolution. The positions of tRNAs in the A and P sites in the pretranslocational complexes and in the P and E sites in the posttranslocational complexes have been determined. Of these, the P-site tRNA position is the same as seen earlier in the initiation-like fMet-tRNA(f)(Met)-ribosome complex, where it was visualized with high accuracy. Now, the positions of the A- and E-site tRNAs are determined with similar accuracy. The positions of the CCA end of the tRNAs at the A site are different before and after peptide bond formation. The relative positions of anticodons of P- and E-site tRNAs in the posttranslocational state are such that a codon-anticodon interaction at the E site appears feasible.
Figures


References
-
- Agrawal R.K., Burma D.P. Sites of ribosomal RNAs involved in the subunit association of tight and loose couple ribosomes. J. Biol. Chem. 1996;271:21285–21291. - PubMed
-
- Agrawal R.K., Frank J. Structural studies of translational apparatus. Curr. Opin. Struct. Biol. 1999;9:215–221. - PubMed
-
- Agrawal R.K., Penczek P., Grassucci R.A., Li Y., Leith A., Nierhaus K.H., Frank J. Direct visualization of A-, P-, and E-site transfer RNAs in the Escherichia coli ribosome. Science. 1996;271:1000–1002. - PubMed
-
- Agrawal R.K., Heagle A.B., Penczek P., Grassucci R.A., Frank J. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome Nat. Struct. Biol 6 1999. 643 647a - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

