Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae
- PMID: 10931860
- PMCID: PMC2175198
- DOI: 10.1083/jcb.150.3.461
Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae
Abstract
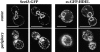
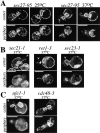
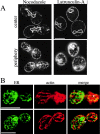
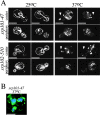
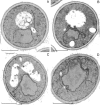
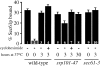
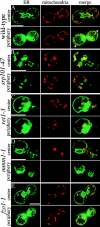
We find that the peripheral ER in Saccharomyces cerevisiae forms a dynamic network of interconnecting membrane tubules throughout the cell cycle, similar to the ER in higher eukaryotes. Maintenance of this network does not require microtubule or actin filaments, but its dynamic behavior is largely dependent on the actin cytoskeleton. We isolated three conditional mutants that disrupt peripheral ER structure. One has a mutation in a component of the COPI coat complex, which is required for vesicle budding. This mutant has a partial defect in ER segregation into daughter cells and disorganized ER in mother cells. A similar phenotype was found in other mutants with defects in vesicular trafficking between ER and Golgi complex, but not in mutants blocked at later steps in the secretory pathway. The other two mutants found in the screen have defects in the signal recognition particle (SRP) receptor. This receptor, along with SRP, targets ribosome-nascent chain complexes to the ER membrane for protein translocation. A conditional mutation in SRP also disrupts ER structure, but other mutants with translocation defects do not. We also demonstrate that, both in wild-type and mutant cells, the ER and mitochondria partially coalign, and that mutations that disrupt ER structure also affect mitochondrial structure. Our data suggest that both trafficking between the ER and Golgi complex and ribosome targeting are important for maintaining ER structure, and that proper ER structure may be required to maintain mitochondrial structure.
Figures




References
-
- Achleitner G., Gaigg B., Krasser A., Kainersdorfer E., Kohlwein S.D., Perktold A., Zellnig G., Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 1999;264:545–553. - PubMed
-
- Amberg D.C., Goldstein A.L., Cole C.N. Isolation and characterization of RAT1an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

