Nicotinic acid adenine dinucleotide phosphate (NAADP(+)) is an essential regulator of T-lymphocyte Ca(2+)-signaling
- PMID: 10931869
- PMCID: PMC2175191
- DOI: 10.1083/jcb.150.3.581
Nicotinic acid adenine dinucleotide phosphate (NAADP(+)) is an essential regulator of T-lymphocyte Ca(2+)-signaling
Abstract
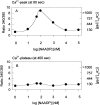
Microinjection of human Jurkat T-lymphocytes with nicotinic acid adenine dinucleotide phosphate (NAADP(+)) dose-dependently stimulated intracellular Ca(2+)-signaling. At a concentration of 10 nM NAADP(+) evoked repetitive and long-lasting Ca(2+)-oscillations of low amplitude, whereas at 50 and 100 nM, a rapid and high initial Ca(2+)-peak followed by trains of smaller Ca(2+)-oscillations was observed. Higher concentrations of NAADP(+) (1 and 10 microM) gradually reduced the initial Ca(2+)-peak, and a complete self-inactivation of Ca(2+)-signals was seen at 100 microM. The effect of NAADP(+) was specific as it was not observed with nicotinamide adenine dinucleotide phosphate. Both inositol 1,4, 5-trisphosphate- and cyclic adenosine diphosphoribose-mediated Ca(2+)-signaling were efficiently inhibited by coinjection of a self-inactivating concentration of NAADP(+). Most importantly, microinjection of a self-inactivating concentration of NAADP(+) completely abolished subsequent stimulation of Ca(2+)-signaling via the T cell receptor/CD3 complex, indicating that a functional NAADP(+) Ca(2+)-release system is essential for T-lymphocyte Ca(2+)-signaling.
Figures





References
-
- Aarhus R., Dickey D.M., Graeff R.M., Gee K.R., Walseth T.F., Lee H.C. Activation and inactivation of Ca2+ release by NAADP+ . J. Biol. Chem. 1996;271:8513–8516. - PubMed
-
- Albrieux M., Lee H.C., Villaz M. Calcium signaling vy cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J. Biol. Chem. 1998;273:14566–14574. - PubMed
-
- Ashamu G.A., Galione A., Potter B.V.L. Chemo-enzymatic synthesis of analogues of the second messenger candidate cyclic adenosine 5′-diphosphate ribose. J. Chem. Soc. Chem. Commun. 1995;1359–1360
-
- Bak J., White P., Timar G., Missiaen L., Genazzani A.A., Galione A. Nicotinic acid adenine dinucleotide phosphate triggers Ca2+ release from brain microsomes. Curr. Biol. 1999;9:751–754. - PubMed
-
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium response curves of Ins(1,4,5)P3 and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

