Characterization of palladin, a novel protein localized to stress fibers and cell adhesions
- PMID: 10931874
- PMCID: PMC2175193
- DOI: 10.1083/jcb.150.3.643
Characterization of palladin, a novel protein localized to stress fibers and cell adhesions
Abstract
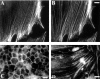
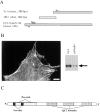
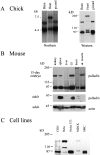
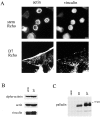
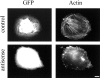
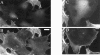
Here, we describe the identification of a novel phosphoprotein named palladin, which colocalizes with alpha-actinin in the stress fibers, focal adhesions, cell-cell junctions, and embryonic Z-lines. Palladin is expressed as a 90-92-kD doublet in fibroblasts and coimmunoprecipitates in a complex with alpha-actinin in fibroblast lysates. A cDNA encoding palladin was isolated by screening a mouse embryo library with mAbs. Palladin has a proline-rich region in the NH(2)-terminal half of the molecule and three tandem Ig C2 domains in the COOH-terminal half. In Northern and Western blots of chick and mouse tissues, multiple isoforms of palladin were detected. Palladin expression is ubiquitous in embryonic tissues, and is downregulated in certain adult tissues in the mouse. To probe the function of palladin in cultured cells, the Rcho-1 trophoblast model was used. Palladin expression was observed to increase in Rcho-1 cells when they began to assemble stress fibers. Antisense constructs were used to attenuate expression of palladin in Rcho-1 cells and fibroblasts, and disruption of the cytoskeleton was observed in both cell types. At longer times after antisense treatment, fibroblasts became fully rounded. These results suggest that palladin is required for the normal organization of the actin cytoskeleton and focal adhesions.
Figures
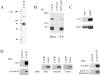




References
-
- Aberle H., Schwartz H., Kemler R. Cadherin–catenin complexprotein interactions and their implication for cadherin function. J. Cell. Biochem. 1996;61:514–523. - PubMed
-
- Arber S., Halder G., Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. - PubMed
-
- Arber S., Hunter J.J., Ross J., Hongo M., Sansig G., Borg J., Perriard J.-C., Chien K.R., Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy and heart failure. Cell. 1997;88:393–403. - PubMed
-
- Beckerle M. Spatial control of actin filament assemblylessons from Listeria . Cell. 1998;95:741–748. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

