Human 100-kDa homologous DNA-pairing protein is the splicing factor PSF and promotes DNA strand invasion
- PMID: 10931916
- PMCID: PMC108454
- DOI: 10.1093/nar/28.16.3022
Human 100-kDa homologous DNA-pairing protein is the splicing factor PSF and promotes DNA strand invasion
Abstract
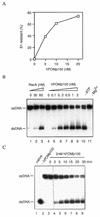
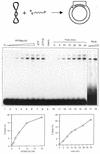
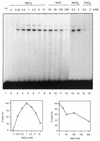
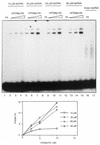
Proteins promoting homologous pairing could be involved in various fundamental biological processes. Previously we detected two mammalian nuclear proteins of 100 and 75 kDa able to promote homologous DNA pairing. Here we report isolation and characterisation of the human (h) 100-kDa DNA-pairing protein, hPOMp100, from HeLa nuclei. The peptide sequences of hPOMp100 revealed identity to the human splicing factor PSF and a DNA-binding subunit of p100/p52 heterodimer of unknown function. Bacterially expressed PSF promotes DNA pairing identical to that of hPOMp100. hPOMp100/PSF binds not only RNA but also both single-stranded (ss) and double-stranded (ds) DNA and facilitates the renaturation of complementary ssDNAs. More important, the protein promotes the incorporation of a ss oligonucleotide into a homologous superhelical dsDNA, D-loop formation. A D-loop is the first heteroduplex DNA intermediate generated between recombining DNA molecules. Moreover, this reaction could be implicated in re-establishing stalled replication forks. Consistent with this hypothesis, DNA-pairing activity of hPOMp100/PSF is associated with cellular proliferation. Significantly, phosphorylation of hPOMp100/PSF by protein kinase C inhibits its binding to RNA but stimulates its binding to DNA and D-loop formation and may represent a regulatory mechanism to direct this multifunctional protein to DNA metabolic pathways.
Figures




References
-
- Roca A.I. and Cox,M.M. (1997) Prog. Nucleic Acid. Res. Mol. Biol., 56, 129–223. - PubMed
-
- Kowalczykowski S.C. and Eggleston,A.K. (1994) Annu. Rev. Biochem., 63, 991–1043. - PubMed
-
- Shinohara A. and Ogawa,T. (1995) Trends Biochem. Sci., 20, 387–391. - PubMed
-
- Roeder G.S. (1997) Genes Dev., 11, 2600–2621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

