Action potentials reliably invade axonal arbors of rat neocortical neurons
- PMID: 10931955
- PMCID: PMC16932
- DOI: 10.1073/pnas.170278697
Action potentials reliably invade axonal arbors of rat neocortical neurons
Abstract
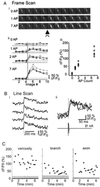
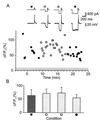
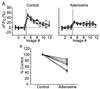
Neocortical pyramidal neurons have extensive axonal arborizations that make thousands of synapses. Action potentials can invade these arbors and cause calcium influx that is required for neurotransmitter release and excitation of postsynaptic targets. Thus, the regulation of action potential invasion in axonal branches might shape the spread of excitation in cortical neural networks. To measure the reliability and extent of action potential invasion into axonal arbors, we have used two-photon excitation laser scanning microscopy to directly image action-potential-mediated calcium influx in single varicosities of layer 2/3 pyramidal neurons in acute brain slices. Our data show that single action potentials or bursts of action potentials reliably invade axonal arbors over a range of developmental ages (postnatal 10-24 days) and temperatures (24 degrees C-30 degrees C). Hyperpolarizing current steps preceding action potential initiation, protocols that had previously been observed to produce failures of action potential propagation in cultured preparations, were ineffective in modulating the spread of action potentials in acute slices. Our data show that action potentials reliably invade the axonal arbors of neocortical pyramidal neurons. Failures in synaptic transmission must therefore originate downstream of action potential invasion. We also explored the function of modulators that inhibit presynaptic calcium influx. Consistent with previous studies, we find that adenosine reduces action-potential-mediated calcium influx in presynaptic terminals. This reduction was observed in all terminals tested, suggesting that some modulatory systems are expressed homogeneously in most terminals of the same neuron.
Figures

Comment in
-
Reliability of axonal propagation: the spike doesn't stop here.Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9349-50. doi: 10.1073/pnas.97.17.9349. Proc Natl Acad Sci U S A. 2000. PMID: 10944204 Free PMC article. No abstract available.
References
-
- Braitenberg V, Schutz A. Anatomy of the Cortex: Studies of Brain Function. Vol. 18. Berlin: Springer; 1991.
-
- Peters A, Palay S L, Webster HD. The Fine Structure of the Nervous System. New York: Oxford Univ. Press; 1991.
-
- Mulkey R M, Zucker R S. Nature (London) 1991;350:153–155. - PubMed
-
- Katz B. The Release of Neurotransmitter Substances. Springfield, IL: Thomas; 1969.
-
- Wall P D. Trends Neurosci. 1995;18:99–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

