Use of reporter genes for optical measurements of neoplastic disease in vivo
- PMID: 10933067
- PMCID: PMC1550286
- DOI: 10.1038/sj.neo.7900079
Use of reporter genes for optical measurements of neoplastic disease in vivo
Abstract
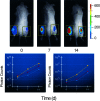
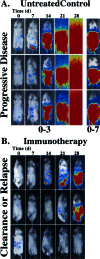
Revealing the cellular and molecular changes associated with cancer, as they occur in intact living animal models of human neoplastic disease, holds tremendous potential for understanding disease mechanisms and elucidating effective therapies. Since light is transmitted through mammalian tissues, at a low level, optical signatures conferred on tumor cells by expression of reporter genes encoding bioluminescent and fluorescent proteins can be detected externally using sensitive photon detection systems. Expression of reporter genes, such as the bioluminescent enzyme firefly luciferase (Luc) or variants of green fluorescent protein (GFP) in transformed cells, can effectively be used to reveal molecular and cellular features of neoplasia in vivo. Tumor cell growth and regression in response to various therapies have been evaluated non-invasively in living experimental animals using these reporter genes. Detection of Luc-labeled cells in vivo was extremely sensitive with signals over background from as few as 1000 human tumor cells distributed throughout the peritoneal cavity of a mouse with linear relationships between cell number and signal intensity over five logs. GFP offers the strength of high-resolution ex vivo analyses following in vivo localization of the tumor. The dynamic range of Luc detection allows the full disease course to be monitored since disease progression from small numbers of cells to extensive disease can be assessed. As such, therapies that target minimal disease as well as those designed for late stage disease can be readily evaluated in animal models. Real time spatiotemporal analyses of tumor cell growth can reveal the dynamics of neoplastic disease, and facilitate rapid optimization of effective treatment regimens. Thus, these methods improve the predictability of animal models of human disease as study groups can be followed over time, and can accelerate the development of therapeutic strategies.
Figures



References
-
- Clemens MJ, Bommer UA. Translational control: the cancer connection. Int J Biochem Cell Biol. 1999;31:1–23. - PubMed
-
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trend Biochem Sci. 1999;24:68–72. - PubMed
-
- Devereux TR, Risinger JI, Barrett JC. Mutations and altered expression of the human cancer genes: what they tell us about causes. IARC Sci Publ. 1999:19–42. - PubMed
-
- Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10–18. - PubMed
-
- Jacobson S, Pillus L. Modifying chromatin and concepts of cancer. Curr Opin Gen Dev. 1999;9:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
