Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer
- PMID: 10933075
- PMCID: PMC1531872
- DOI: 10.1038/sj.neo.7900081
Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer
Abstract
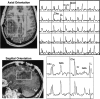
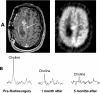
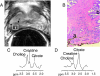
Clinical applications of magnetic resonance spectroscopic imaging (MRSI) for the study of brain and prostate cancer have expanded significantly over the past 10 years. Proton MRSI studies of the brain and prostate have demonstrated the feasibility of noninvasively assessing human cancers based on metabolite levels before and after therapy in a clinically reasonable amount of time. MRSI provides a unique biochemical "window" to study cellular metabolism noninvasively. MRSI studies have demonstrated dramatic spectral differences between normal brain tissue (low choline and high N-acetyl aspartate, NAA) and prostate (low choline and high citrate) compared to brain (low NAA, high choline) and prostate (low citrate, high choline) tumors. The presence of edema and necrosis in both the prostate and brain was reflected by a reduction of the intensity of all resonances due to reduced cell density. MRSI was able to discriminate necrosis (absence of all metabolites, except lipids and lactate) from viable normal tissue and cancer following therapy. The results of current MRSI studies also provide evidence that the magnitude of metabolic changes in regions of cancer before therapy as well as the magnitude and time course of metabolic changes after therapy can improve our understanding of cancer aggressiveness and mechanisms of therapeutic response. Clinically, combined MRI/MRSI has already demonstrated the potential for improved diagnosis, staging and treatment planning of brain and prostate cancer. Additionally, studies are under way to determine the accuracy of anatomic and metabolic parameters in providing an objective quantitative basis for assessing disease progression and response to therapy.
Figures
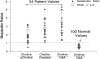







Similar articles
-
Spectroscopy in prostate cancer: hope or hype?Oncology (Williston Park). 2001 Nov;15(11):1399-410; discussion 1415-6, 1418. Oncology (Williston Park). 2001. PMID: 11758871 Review.
-
Prostate cancer: value of magnetic resonance spectroscopy 3D chemical shift imaging.Abdom Imaging. 2006 Jul-Aug;31(4):490-9. doi: 10.1007/s00261-006-9029-8. Abdom Imaging. 2006. PMID: 16955379 Review.
-
Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer.J Magn Reson Imaging. 2002 Oct;16(4):451-63. doi: 10.1002/jmri.10172. J Magn Reson Imaging. 2002. PMID: 12353259 Free PMC article. Review.
-
Time-dependent effects of hormone-deprivation therapy on prostate metabolism as detected by combined magnetic resonance imaging and 3D magnetic resonance spectroscopic imaging.Magn Reson Med. 2001 Jul;46(1):49-57. doi: 10.1002/mrm.1159. Magn Reson Med. 2001. PMID: 11443710
-
Magnetic resonance imaging and spectroscopic imaging of prostate cancer.J Urol. 2003 Dec;170(6 Pt 2):S69-75; discussion S75-6. doi: 10.1097/01.ju.0000094958.23276.c4. J Urol. 2003. PMID: 14610414 Review.
Cited by
-
Compressive sensing could accelerate 1H MR metabolic imaging in the clinic.Radiology. 2012 Mar;262(3):985-94. doi: 10.1148/radiol.11111098. Radiology. 2012. PMID: 22357898 Free PMC article.
-
In vivo lactate signal enhancement using binomial spectral-selective pulses in selective MQ coherence (SS-SelMQC) spectroscopy.Magn Reson Med. 2009 Sep;62(3):591-8. doi: 10.1002/mrm.22065. Magn Reson Med. 2009. PMID: 19526486 Free PMC article.
-
Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment.PLoS One. 2009 Nov 12;4(11):e7819. doi: 10.1371/journal.pone.0007819. PLoS One. 2009. PMID: 19915674 Free PMC article.
-
Multimodal mass spectrometric imaging of small molecules reveals distinct spatio-molecular signatures in differentially metastatic breast tumor models.Cancer Res. 2010 Nov 15;70(22):9012-21. doi: 10.1158/0008-5472.CAN-10-0360. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045154 Free PMC article.
-
Metabolomics: a novel approach to early and noninvasive prostate cancer detection.Korean J Urol. 2011 Feb;52(2):79-89. doi: 10.4111/kju.2011.52.2.79. Epub 2011 Feb 19. Korean J Urol. 2011. PMID: 21379423 Free PMC article.
References
-
- Arnold DL, Shoubridge EA, Feindel W, Villemure JG. Metabolic changes in cerebral gliomas within hours of treatment with intra-arterial BCNU demonstrated by phosphorus magnetic resonance spectroscopy. Can J Neurol Sci. 1987;14:570–575. - PubMed
-
- Arnold DL, Shoubridge EA, Emrich J, Feindel W, Villemure JG. Early metabolic changes following chemotherapy of human gliomas in vivo demonstrated by phosphorus magnetic resonance spectroscopy. Invest Radiol. 1989;24:958–961. - PubMed
-
- Arnold DL, Shoubridge EA, Villemure JG, Feindel W. Proton and phosphorus magnetic resonance spectroscopy of human astrocytomas in vivo. Preliminary observations on tumor grading. NMR Biomed. 1990;3:184–189. - PubMed
-
- Arnold DL, Emrich JF, Shoubridge EA, Villemure JG, Feindel W. Characterization of astrocytomas, meningiomas, and pituitary adenomas by phosphorus magnetic resonance spectroscopy. J Neurosurg. 1991;74:447–453. - PubMed
-
- Arnold DL, De Stefano N. Magnetic resonance spectroscopy in vivo: applications in neurological disorders. Ital J Neurol Sci. 1997;18:321–329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
