Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2
- PMID: 10933704
- PMCID: PMC112327
- DOI: 10.1128/jvi.74.17.7963-7971.2000
Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2
Abstract
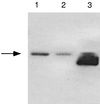
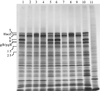
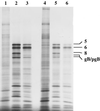
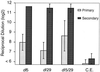
A number of studies have shown that replication-defective mutant strains of herpes simplex virus (HSV) can induce protective immunity in animal systems against wild-type HSV challenge. However, all of those studies used viruses with single mutations. Because multiple, stable mutations provide optimal levels of safety for live vaccines, we felt that additional mutations needed to be engineered into a candidate vaccine strain for HSV-2 and genital herpes. We therefore isolated an HSV-2 strain with deletion mutations in two viral DNA replication protein genes, UL5 and UL29. The resulting double deletion mutant virus strain, dl5-29, fails to form plaques or to give any detectable single cycle yields in normal monkey or human cells. Nevertheless, dl5-29 expresses nearly the same pattern of gene products as the wild-type virus or the single mutant viruses and induces antibody titers in mice that are equivalent to those induced by single deletion mutant viruses. Therefore, it is feasible to isolate a mutant HSV strain with two mutations in essential genes and with an increased level of safety but which is still highly immunogenic.
Figures


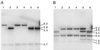

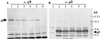
Similar articles
-
Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection.Virology. 2001 Sep 30;288(2):256-63. doi: 10.1006/viro.2001.1094. Virology. 2001. PMID: 11601897
-
Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease.Virology. 1997 May 26;232(1):1-12. doi: 10.1006/viro.1997.8564. Virology. 1997. PMID: 9185583
-
Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs.Vaccine. 2008 Jul 29;26(32):4034-40. doi: 10.1016/j.vaccine.2008.05.022. Epub 2008 Jun 2. Vaccine. 2008. PMID: 18565628 Free PMC article.
-
Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs.J Virol. 2005 Jan;79(1):410-8. doi: 10.1128/JVI.79.1.410-418.2005. J Virol. 2005. PMID: 15596834 Free PMC article.
-
Herpes Simplex Viruses Whose Replication Can Be Deliberately Controlled as Candidate Vaccines.Vaccines (Basel). 2020 May 18;8(2):230. doi: 10.3390/vaccines8020230. Vaccines (Basel). 2020. PMID: 32443425 Free PMC article. Review.
Cited by
-
Alphaherpesvirus Vaccines.Curr Issues Mol Biol. 2021;41:469-508. doi: 10.21775/cimb.041.469. Epub 2020 Sep 23. Curr Issues Mol Biol. 2021. PMID: 32963118 Free PMC article. Review.
-
Virus-specific immune memory at peripheral sites of herpes simplex virus type 2 (HSV-2) infection in guinea pigs.PLoS One. 2014 Dec 8;9(12):e114652. doi: 10.1371/journal.pone.0114652. eCollection 2014. PLoS One. 2014. PMID: 25485971 Free PMC article.
-
Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge.J Virol. 2015 Jan;89(1):83-96. doi: 10.1128/JVI.02380-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320297 Free PMC article.
-
Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein.Vaccine. 2010 Mar 24;28(15):2754-62. doi: 10.1016/j.vaccine.2010.01.030. Epub 2010 Jan 29. Vaccine. 2010. PMID: 20117270 Free PMC article.
-
Herpes simplex virus infected cell protein 8 is required for viral inhibition of the cGAS pathway.Virology. 2023 Aug;585:34-41. doi: 10.1016/j.virol.2023.05.002. Epub 2023 May 25. Virology. 2023. PMID: 37271042 Free PMC article.
References
-
- Augenbraun M H, McCormack W M. Sexually transmitted diseases in HIV-infected persons. Infect Dis Clin North Am. 1994;8:439–448. - PubMed
-
- Bernstein D I, Stanberry L R. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–1689. - PubMed
-
- Boursnell M E G, Entwisle C, Blakeley D, Roberts C, Duncan I A, Chisholm S E, Martin G M, Jennings R, Ni Challanain D, Sobek I, Inglis S C, McLean C S. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175:16–25. - PMC - PubMed
-
- Brubaker J O, Thompson C M, Morrison L A, Knipe D M, Siber G B, Finberg R W. Th1-associated immune responses to beta-galactosidase expressed by replication-defective herpes simplex virus. J Immunol. 1996;157:1598–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

