Targeted infection of endothelial cells by avian influenza virus A/FPV/Rostock/34 (H7N1) in chicken embryos
- PMID: 10933711
- PMCID: PMC112334
- DOI: 10.1128/jvi.74.17.8018-8027.2000
Targeted infection of endothelial cells by avian influenza virus A/FPV/Rostock/34 (H7N1) in chicken embryos
Abstract
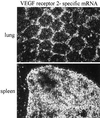
The tissue tropism and spread of infection of the highly pathogenic avian influenza virus A/FPV/Rostock/34 (H7N1) (FPV) were analyzed in 11-day-old chicken embryos. As shown by in situ hybridization, the virus caused generalized infection that was strictly confined to endothelial cells in all organs. Studies with reassortants of FPV and the apathogenic avian strain A/chick/Germany/N/49 (H10N7) revealed that endotheliotropism was linked to FPV hemagglutinin (HA). To further analyze the factors determining endotheliotropism, the HA-activating protease furin was cloned from chicken tissue. Ubiquitous expression of furin and other proprotein convertases in the chick embryo indicated that proteolytic activation of HA was not responsible for restriction of infection to the endothelium. To determine the expression of virus receptors in embryonic tissues, histochemical analysis of alpha2,3- and alpha2,6-linked neuraminic acid was carried out by lectin-binding assays. These receptors were found on endothelial cells and on several epithelial cells, but not on tissues surrounding endothelia. Finally, we analyzed the polarity of virus maturation in endothelial cells. Studies on cultured human endothelial cells employing confocal laser scanning microscopy revealed that HA is specifically targeted to the apical surface of these cells, and electron microscopy of embryonic tissues showed that virus maturation occurs also at the luminar side. Taken together, these observations indicate that endotheliotropism of FPV in the chicken embryo is determined, on one hand, by the high cleavability of HA, which mediates virus entry into the vascular system, and, on the other hand, by restricted receptor expression and polar budding, which prevent spread of infection into tissues surrounding endothelia.
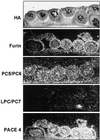
Figures






Similar articles
-
Proprotein-processing endoproteases PC6 and furin both activate hemagglutinin of virulent avian influenza viruses.J Virol. 1994 Sep;68(9):6074-8. doi: 10.1128/JVI.68.9.6074-6078.1994. J Virol. 1994. PMID: 8057485 Free PMC article.
-
The NS segment of an H5N1 highly pathogenic avian influenza virus (HPAIV) is sufficient to alter replication efficiency, cell tropism, and host range of an H7N1 HPAIV.J Virol. 2010 Feb;84(4):2122-33. doi: 10.1128/JVI.01668-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007264 Free PMC article.
-
Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):375-83. doi: 10.1016/j.ejcb.2015.05.013. Epub 2015 Jun 1. Eur J Cell Biol. 2015. PMID: 26095298
-
The hemagglutinin: a determinant of pathogenicity.Curr Top Microbiol Immunol. 2014;385:3-34. doi: 10.1007/82_2014_384. Curr Top Microbiol Immunol. 2014. PMID: 25031010 Review.
-
Infection of the endothelium by influenza viruses.Thromb Haemost. 2005 Aug;94(2):262-5. doi: 10.1160/TH05-04-0264. Thromb Haemost. 2005. PMID: 16113814 Review.
Cited by
-
Venous thromboembolism in viral diseases: A comprehensive literature review.Health Sci Rep. 2023 Feb 5;6(2):e1085. doi: 10.1002/hsr2.1085. eCollection 2023 Feb. Health Sci Rep. 2023. PMID: 36778773 Free PMC article.
-
Restrictions to the adaptation of influenza a virus h5 hemagglutinin to the human host.J Virol. 2004 Jan;78(1):502-7. doi: 10.1128/jvi.78.1.502-507.2004. J Virol. 2004. PMID: 14671130 Free PMC article.
-
Neuropathogenesis of a highly pathogenic avian influenza virus (H7N1) in experimentally infected chickens.Vet Res. 2011 Oct 7;42(1):106. doi: 10.1186/1297-9716-42-106. Vet Res. 2011. PMID: 21982125 Free PMC article.
-
Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection.Virulence. 2013 Aug 15;4(6):537-42. doi: 10.4161/viru.25779. Epub 2013 Jul 17. Virulence. 2013. PMID: 23863601 Free PMC article. Review.
-
A viral race for primacy: co-infection of a natural pair of low and highly pathogenic H7N7 avian influenza viruses in chickens and embryonated chicken eggs.Emerg Microbes Infect. 2018 Dec 5;7(1):204. doi: 10.1038/s41426-018-0204-0. Emerg Microbes Infect. 2018. PMID: 30514922 Free PMC article.
References
-
- Banks J, Speidel E, Alexander D J. Characterization of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch Virol. 1998;143:781–787. - PubMed
-
- Beaubien G, Schafer M K, Weihe E, Dong W, Chretien M, Seidah N G, Day R. The distinct gene expression of the pro-hormone convertases in the rat heart suggests potential substrates. Cell Tissue Res. 1995;279:539–549. - PubMed
-
- Campan M, Yoshizumi M, Seidah N G, Lee M E, Bianchi C, Haber E. Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry. 1996;35:3797–3802. - PubMed
-
- Codogno P, Aubery M. Changes in cell-surface sialic acid content during chick embryo development. Mech Ageing Dev. 1983;23:307–314. - PubMed
-
- Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

