Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo
- PMID: 10934148
- PMCID: PMC1850124
- DOI: 10.1016/S0002-9440(10)64556-7
Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo
Abstract
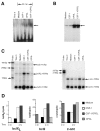
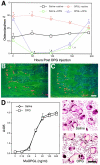
Osteoprotegerin ligand (OPGL) targets osteoclast precursors and osteoclasts to enhance differentiation and activation, however, little is known about OPGL effects on osteoclast survival. In vitro, the combination of OPGL + colony-stimulating factor-1 (CSF-1) is required for optimal osteoclast survival. Ultrastructurally, apoptotic changes were observed in detached cells and culture lysates exhibited elevated caspase 3 activity, particularly in cultures lacking CSF-1. DEVD-FMK (caspase 3 inhibitor) partially protected cells when combined with OPGL, but not when used alone or in combination with CSF-1. CSF-1 maintained NF-kappaB activation and increased the expression of bcl-2 and bcl-X(L) mRNA, but had no effect on JNK activation. In contrast, OPGL enhanced both NF-kappaB and JNK kinase activation and increased the expression of c-src, but not bcl-2 and bcl-X(L) mRNA. These data suggest that aspects of both OPGL's and CSF-1's signaling/survival pathways are required for optimal osteoclast survival. In mice, a single dose of OPG, the OPGL decoy receptor, led to a >90% loss of osteoclasts because of apoptosis within 48 hours of exposure without impacting osteoclast precursor cells. Therefore, OPGL is essential, but not sufficient, for osteoclast survival and endogenous CSF-1 levels are insufficient to maintain osteoclast viability in the absence of OPGL.
Figures






References
-
- Chambers TJ: The cellular basis of bone resorption. Clin Orthop 1980, 151:283-293 - PubMed
-
- Rodan GA, Martin TJ: Role of osteoblasts in hormonal control of bone resorption. Calcif Tissue Int 1981, 33:349-351 - PubMed
-
- Lacey DL, Timms E, Tan H-L, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian Y-X, Kaufman S, Sarosi I, Shalhoub V, Senaldi V, Guo G, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 - PubMed
-
- Shalhoub V, Faust J, Boyle WJ, Dunstan CR, Kelley M, Kaufman S, Scully S, Van G, Lacey DL: Osteoprotegerin and osteoprotegerin effects on osteoclast formation from peripheral blood mononuclear cell precursors. J Cell Biochem 1999, 72:251-261 - PubMed
-
- Yoshida H, Hayashi S-I, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S-I: The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345:442-444 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

