Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin
- PMID: 10934155
- PMCID: PMC1850142
- DOI: 10.1016/S0002-9440(10)64563-4
Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin
Abstract
Increased expression of matrix metalloproteinases, particularly gelatinase B (MMP-9), has been described in the lungs in pulmonary fibrosis. Intratracheal bleomycin is often used experimentally to produce lesions resembling human fibrosing alveolitis. To assess the role of gelatinase B in bleomycin-induced fibrosing alveolitis, we instilled bleomycin intratracheally into gelatinase B-deficient mice and gelatinase B+/+ littermates. Twenty-one days after bleomycin the two groups of mice were indistinguishable in terms of pulmonary histology and total lung collagen and elastin. However, the lungs of gelatinase B-deficient mice showed minimal alveolar bronchiolization, whereas bronchiolization was prominent in the lungs of gelatinase B+/+ mice. Gelatinase B was identified immunohistochemically in terminal bronchiolar cells and bronchiolized cells 7 and 14 days after bleomycin in gelatinase B+/+ mice, and whole lung gelatinase B mRNA was increased at the same times. Many bronchiolized cells displayed Clara cell features by electron microscopy. Some bronchiolized cells stained with antibody to helix transcription factor 4, a factor associated with the ciliated cell phenotype. Thus, fibrosing alveolitis develops after intratracheal bleomycin irrespective of gelatinase B. However, gelatinase B is required for alveolar bronchiolization, perhaps by facilitating migration of Clara cells and other bronchiolar cells into the regions of alveolar injury.
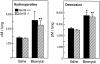
Figures







References
-
- Woessner JF Jr: The matrix metalloproteinase family. Edited by WC Parks, RP Mecham. San Diego, Academic Press, 1998, pp 1–14
-
- Shapiro SD, Senior RM: Matrix metalloproteinases. Matrix degradation and more. Am J Respir Cell Mol Biol 1999, 20:1100-1102 - PubMed
-
- Vu TH, Werb Z: Gelatinase B: structure, regulation, and function. Parks WC Mecham RP eds. Matrix Metalloproteinases. 1998, :pp 115-148 Academic Press, San Diego
-
- Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrance VJ, Travis WD: Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 1996, 149:1241-1256 - PMC - PubMed
-
- Fukuda Y, Ishizuka M, Kudoh S, Kitaichi M, Yamanaka N: Localization of matrix metalloproteinase-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 1998, 78:687-698 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

