Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis
- PMID: 10934231
- PMCID: PMC2193219
- DOI: 10.1084/jem.192.3.433
Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis
Abstract
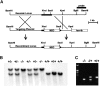
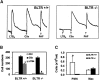
Leukotrienes are derived from arachidonic acid and serve as mediators of inflammation and immediate hypersensitivity. Leukotriene B(4) (LTB(4)) and leukotriene C(4) (LTC(4)) act through G protein-coupled receptors LTB(4) receptor (BLTR) and Cys-LTR, respectively. To investigate the physiological role of BLTR, we produced mice with a targeted disruption of the BLTR gene. Mice deficient for BLTR (BLTR(-/-)) developed normally and had no apparent hematopoietic abnormalities. Peritoneal neutrophils from BLTR(-/-) mice displayed normal responses to the inflammatory mediators C5a and platelet-activating factor (PAF) but did not respond to LTB(4) for calcium mobilization or chemotaxis. Additionally, LTB(4) elicited peritoneal neutrophil influx in control but not in BLTR(-/-) mice. Thus, BLTR is the sole receptor for LTB(4)-induced inflammation in mice. Neutrophil influx in a peritonitis model and acute ear inflammation in response to arachidonic acid was significantly reduced in BLTR(-/-) mice. In mice, intravenous administration of PAF induces immediate lethal anaphylaxis. Surprisingly, female BLTR(-/-) mice displayed selective survival (6 of 9; P = 0.002) relative to male (1 of 11) mice of PAF-induced anaphylaxis. These results demonstrate the role of BLTR in leukotriene-mediated acute inflammation and an unexpected sex-related involvement in PAF-induced anaphylaxis.
Figures



Comment in
-
The scent of a phagocyte: Advances on leukotriene b(4) receptors.J Exp Med. 2000 Aug 7;192(3):F5-8. doi: 10.1084/jem.192.3.f5. J Exp Med. 2000. PMID: 10934235 Free PMC article. Review. No abstract available.
References
-
- Smith M.J., Ford-Hutchinson A.W., Bray M.A. Leukotriene Ba potential mediator of inflammation. J. Pharm. Pharmacol. 1980;32:517–518. - PubMed
-
- Samuelsson B., Dahlen S.E., Lindgren J.A., Rouzer C.A., Serhan C.N. Leukotrienes and lipoxinsstructures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. - PubMed
-
- Dahlen S.E., Hedqvist P., Hammarstrom S., Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–486. - PubMed
-
- Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. A G-protein-coupled receptor for leukotriene B-4 that mediates chemotaxis. Nature. 1997;387:620–624. - PubMed
-
- Lynch K.R., O'Neill G.P., Liu Q.Y., Im D.S., Sawyer N., Metters K.M., Coulombe N., Abramovitz M., Figueroa D.J., Zeng Z.Z. Characterization of the human cysteinyl leukotriene CysLT(1) receptor. Nature. 1999;399:789–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

