Activating Ly-49D and inhibitory Ly-49A natural killer cell receptors demonstrate distinct requirements for interaction with H2-D(d)
- PMID: 10934233
- PMCID: PMC2193226
- DOI: 10.1084/jem.192.3.447
Activating Ly-49D and inhibitory Ly-49A natural killer cell receptors demonstrate distinct requirements for interaction with H2-D(d)
Abstract
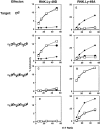
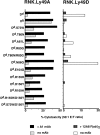
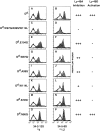
The activating Ly-49D receptor and the inhibitory Ly-49A receptor mediate opposing effects on natural killer (NK) cell cytotoxicity after interaction with the same major histocompatibility complex ligand, H2-D(d). To compare Ly-49D and Ly-49A interactions with H2-D(d), we created mutations in H2-D(d) and examined the functional ability of these mutants to activate lysis through Ly-49D or to inhibit lysis through Ly-49A. Specific single amino acid changes in either the H2-D(d) alpha(1) helix or the alpha(2) helix abrogated Ly-49D-mediated cytotoxicity, but these changes had no significant effect on Ly-49A-dependent inhibition. Each of three alpha(2) domain mutations in the floor of the peptide binding groove reduced functional recognition by either Ly-49D or Ly-49A, but all three were required to fully abrogate inhibition by Ly-49A. Our studies indicate that Ly-49D/H2-D(d) interactions require distinct determinants compared with Ly-49A/H2-D(d) interactions. These differences have important implications for the integration of activating and inhibitory signals in NK cells.
Figures


Similar articles
-
Ligand interactions by activating and inhibitory Ly-49 receptors.Immunol Rev. 2001 Jun;181:138-48. doi: 10.1034/j.1600-065x.2001.1810111.x. Immunol Rev. 2001. PMID: 11513135 Review.
-
Synergistic effects of in vivo depletion of Ly-49A and Ly-49G2 natural killer cell subsets in the rejection of H2(b) bone marrow cell allografts.Blood. 2000 Jun 15;95(12):3840-4. Blood. 2000. PMID: 10845918
-
Mouse Ly-49D recognizes H-2Dd and activates natural killer cell cytotoxicity.J Exp Med. 1999 Feb 1;189(3):493-500. doi: 10.1084/jem.189.3.493. J Exp Med. 1999. PMID: 9927511 Free PMC article.
-
The lectin-like NK cell receptor Ly-49A recognizes a carbohydrate-independent epitope on its MHC class I ligand.Immunity. 1998 Feb;8(2):245-54. doi: 10.1016/s1074-7613(00)80476-8. Immunity. 1998. PMID: 9492005
-
A family of murine NK cell receptors specific for target cell MHC class I molecules.Semin Immunol. 1995 Apr;7(2):89-101. doi: 10.1006/smim.1995.0013. Semin Immunol. 1995. PMID: 7579199 Review.
Cited by
-
Natural killer cell tolerance persists despite significant reduction of self MHC class I on normal target cells in mice.PLoS One. 2010 Oct 4;5(10):e13174. doi: 10.1371/journal.pone.0013174. PLoS One. 2010. PMID: 20957233 Free PMC article.
-
The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand.J Exp Med. 2001 Jan 15;193(2):147-58. doi: 10.1084/jem.193.2.147. J Exp Med. 2001. PMID: 11148219 Free PMC article.
-
H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function.J Exp Med. 2001 Nov 19;194(10):1531-9. doi: 10.1084/jem.194.10.1531. J Exp Med. 2001. PMID: 11714759 Free PMC article.
-
Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules.J Exp Med. 2005 Apr 4;201(7):1145-55. doi: 10.1084/jem.20050167. J Exp Med. 2005. PMID: 15809355 Free PMC article.
-
NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy.Cancer Immunol Immunother. 2004 Jan;53(1):41-52. doi: 10.1007/s00262-003-0435-2. Epub 2003 Sep 18. Cancer Immunol Immunother. 2004. PMID: 14504825 Free PMC article.
References
-
- Lanier L.L. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. - PubMed
-
- Yokoyama W.M., Daniels B.F., Seaman W.E., Hunziker R., Margulies D.H., Smith H.R. A family of murine NK cell receptors specific for target cell MHC class I molecules. Semin. Immunol. 1995;7:89–101. - PubMed
-
- Takei F., Brennan J., Mager D.L. The Ly-49 familygenes, proteins and recognition of class I MHC. Immunol. Rev. 1997;155:67–77. - PubMed
-
- Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. - PubMed
-
- George T.C., Mason L.H., Ortaldo J.R., Kumar V., Bennett M. Positive recognition of MHC class I molecules by the Ly49D receptor of murine NK cells. J. Immunol. 1999;162:2035–2043. - PubMed

