Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses
- PMID: 10934236
- PMCID: PMC6772602
- DOI: 10.1523/JNEUROSCI.20-16-05899.2000
Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses
Abstract
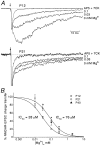
During cerebellar development, granule cells display well characterized changes in the expression of NMDA receptor (NMDAR) NR2 subunits, switching from NR2B to NR2A and NR2C in mature cells. Although various studies, including experiments on mutant mice with one or more NR2 subunit types deleted, suggest that NR2A, NR2B, and NR2C subunits contribute to synaptic NMDARs, changes in the properties of the mossy fiber EPSC during development have not been fully evaluated. In particular, information on NMDAR EPSCs in mature animals is lacking. We have examined pharmacological and kinetic properties of NMDARs at mossy fiber-granule cell synapses from their formation to maturity [postnatal day 7 (P7)-P40 rats]. Significant changes were seen in the relative amplitudes of the non-NMDAR- and NMDAR-mediated components of the evoked EPSC and in the decay kinetics of the latter. The NMDA/non-NMDA ratio was similar at P7, P21, and P40, but showed a clear peak at P12. This change coincided with a speeding of the NMDAR EPSC decay, accompanied by a decrease in sensitivity to ifenprodil (selective NR2B-antagonist). By P21, sensitivity of the NMDAR EPSC to Mg(2+) was approximately threefold less than that at P12 (IC(50), 76 vs 28 microm), suggesting incorporation of the NR2C subunit. However, the predicted slowing of decay kinetics to a value more characteristic of NR2C deactivation, was not seen until P40. Our data are consistent with the known switch from NR2B to NR2A subunits during the first two postnatal weeks, but suggest a gradual incorporation of the NR2C subunit that modifies Mg(2+) sensitivity and only later influences EPSC kinetics.
Figures




References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. - PubMed
-
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. - PubMed
-
- Chazot PL, Coleman SK, Cik M, Stephenson FA. Molecular characterisation of N-methyl-d-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem. 1994;269:24403–24409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
