The Ets transcription factor GABP is required for postsynaptic differentiation in vivo
- PMID: 10934247
- PMCID: PMC6772583
- DOI: 10.1523/JNEUROSCI.20-16-05989.2000
The Ets transcription factor GABP is required for postsynaptic differentiation in vivo
Abstract
At chemical synapses, neurotransmitter receptors are concentrated in the postsynaptic membrane. During the development of the neuromuscular junction, motor neurons induce aggregation of acetylcholine receptors (AChRs) underneath the nerve terminal by the redistribution of existing AChRs and preferential transcription of the AChR subunit genes in subsynaptic myonuclei. Neural agrin, when expressed in nonsynaptic regions of muscle fibers in vivo, activates both mechanisms resulting in the assembly of a fully functional postsynaptic apparatus. Several lines of evidence indicate that synaptic transcription of AChR genes is primarily dependent on a promoter element called N-box. The Ets-related transcription factor growth-associated binding protein (GABP) binds to this motif and has thus been suggested to regulate synaptic gene expression. Here, we assessed the role of GABP in synaptic gene expression and in the formation of postsynaptic specializations in vivo by perturbing its function during postsynaptic differentiation induced by neural agrin. We find that neural agrin-mediated activation of the AChR epsilon subunit promoter is abolished by the inhibition of GABP function. Importantly, the number of AChR aggregates formed in response to neural agrin was strongly reduced. Moreover, aggregates of acetylcholine esterase and utrophin, two additional components of the postsynaptic apparatus, were also reduced. Together, these results are the first direct in vivo evidence that GABP regulates synapse-specific gene expression at the neuromuscular junction and that GABP is required for the formation of a functional postsynaptic apparatus.
Figures



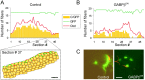
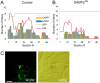
Similar articles
-
A novel pathway for MuSK to induce key genes in neuromuscular synapse formation.J Cell Biol. 2003 May 26;161(4):727-36. doi: 10.1083/jcb.200210156. Epub 2003 May 19. J Cell Biol. 2003. PMID: 12756238 Free PMC article.
-
Synapse-specific gene expression at the neuromuscular junction.Ann N Y Acad Sci. 2003 Sep;998:53-65. doi: 10.1196/annals.1254.008. Ann N Y Acad Sci. 2003. PMID: 14592863 Review.
-
Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse.Dev Neurobiol. 2008 Aug;68(9):1153-69. doi: 10.1002/dneu.20654. Dev Neurobiol. 2008. PMID: 18506821
-
A minigene of neural agrin encoding the laminin-binding and acetylcholine receptor-aggregating domains is sufficient to induce postsynaptic differentiation in muscle fibres.Eur J Neurosci. 1998 Oct;10(10):3141-52. doi: 10.1046/j.1460-9568.1998.00320.x. Eur J Neurosci. 1998. PMID: 9786208
-
Development of the neuromuscular junction.Cell Tissue Res. 2006 Nov;326(2):263-71. doi: 10.1007/s00441-006-0237-x. Epub 2006 Jul 4. Cell Tissue Res. 2006. PMID: 16819627 Review.
Cited by
-
Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference.EMBO Rep. 2004 Feb;5(2):183-8. doi: 10.1038/sj.embor.7400065. Epub 2004 Jan 16. EMBO Rep. 2004. PMID: 14749715 Free PMC article.
-
Targeting of the ETS factor GABPalpha disrupts neuromuscular junction synaptic function.Mol Cell Biol. 2007 May;27(9):3470-80. doi: 10.1128/MCB.00659-06. Epub 2007 Feb 26. Mol Cell Biol. 2007. PMID: 17325042 Free PMC article.
-
ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control.J Neurosci. 2003 Jun 1;23(11):4445-56. doi: 10.1523/JNEUROSCI.23-11-04445.2003. J Neurosci. 2003. PMID: 12805285 Free PMC article.
-
GABP Promotes Mesangial Cell Proliferation and Renal Fibrosis Through GLI1 in Diabetic Nephropathy.Adv Sci (Weinh). 2025 Apr;12(15):e2407462. doi: 10.1002/advs.202407462. Epub 2025 Feb 22. Adv Sci (Weinh). 2025. PMID: 39985381 Free PMC article.
-
A novel pathway for MuSK to induce key genes in neuromuscular synapse formation.J Cell Biol. 2003 May 26;161(4):727-36. doi: 10.1083/jcb.200210156. Epub 2003 May 19. J Cell Biol. 2003. PMID: 12756238 Free PMC article.
References
-
- Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signalling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. - PubMed
-
- Batchelor AH, Piper DE, de la Brousse FC, McKnight SL, Wolberger C. The structure of GABPalpha/beta: an ETS domain- ankyrin repeat heterodimer bound to DNA. Science. 1998;279:1037–1041. - PubMed
-
- Bowen DC, Park JS, Bodine S, Stark JL, Valenzuela DM, Stitt TN, Yancopoulos GD, Lindsay RM, Glass DJ, DiStefano PS. Localization and regulation of MuSK at the neuromuscular junction. Dev Biol. 1998;199:309–319. - PubMed
-
- Brenner HR, Witzemann V, Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990;344:544–547. - PubMed
-
- Brown TA, McKnight SL. Specificities of protein–protein and protein–DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992;6:2502–2512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
