Neuropathology in mice expressing human alpha-synuclein
- PMID: 10934251
- PMCID: PMC6772584
- DOI: 10.1523/JNEUROSCI.20-16-06021.2000
Neuropathology in mice expressing human alpha-synuclein
Abstract
The presynaptic protein alpha-synuclein is a prime suspect for contributing to Lewy pathology and clinical aspects of diseases, including Parkinson's disease, dementia with Lewy bodies, and a Lewy body variant of Alzheimer's disease. alpha-Synuclein accumulates in Lewy bodies and Lewy neurites, and two missense mutations (A53T and A30P) in the alpha-synuclein gene are genetically linked to rare familial forms of Parkinson's disease. Under control of mouse Thy1 regulatory sequences, expression of A53T mutant human alpha-synuclein in the nervous system of transgenic mice generated animals with neuronal alpha-synucleinopathy, features strikingly similar to those observed in human brains with Lewy pathology, neuronal degeneration, and motor defects, despite a lack of transgene expression in dopaminergic neurons of the substantia nigra pars compacta. Neurons in brainstem and motor neurons appeared particularly vulnerable. Motor neuron pathology included axonal damage and denervation of neuromuscular junctions in several muscles examined, suggesting that alpha-synuclein interfered with a universal mechanism of synapse maintenance. Thy1 transgene expression of wild-type human alpha-synuclein resulted in similar pathological changes, thus supporting a central role for mutant and wild-type alpha-synuclein in familial and idiotypic forms of diseases with neuronal alpha-synucleinopathy and Lewy pathology. These mouse models provide a means to address fundamental aspects of alpha-synucleinopathy and test therapeutic strategies.
Figures
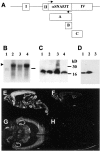
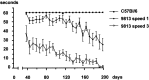




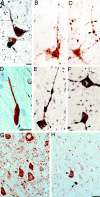

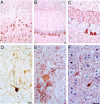
References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neuronal growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Arai T, Ueda K, Ikeda K, Akiyama H, Haga C, Kondo H, Kuroki N, Niizato K, Iritani S, Tsuchiya K. Argyrophilic glial inclusions in the midbrain of patients with Parkinson's disease and diffuse Lewy body disease are immunopositive for NACP/alpha-synuclein. Neurosci Lett. 1999;259:83–86. - PubMed
-
- Arima K, Uéda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M. Immunoelectron microscopic demonstration of NACP/α-synuclein-epitopes on the filamentous components of Lewy bodies in Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. - PubMed
-
- Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
